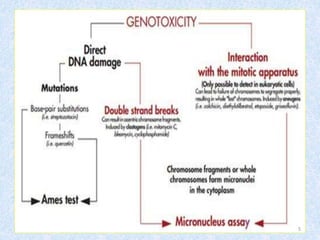
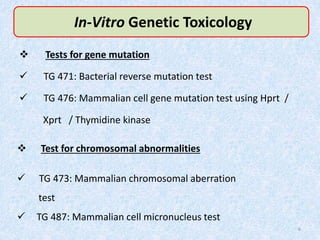
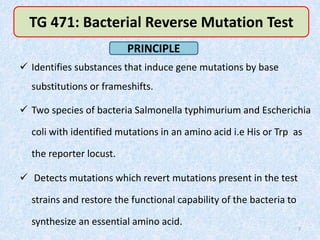
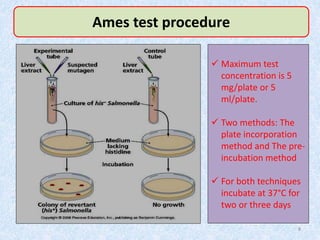
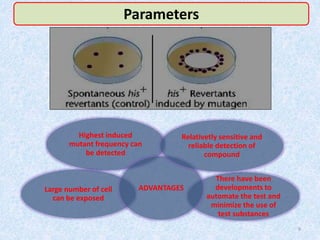
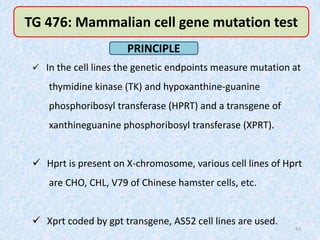
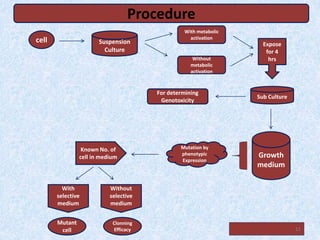
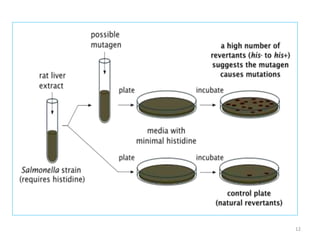
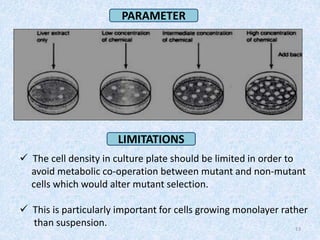
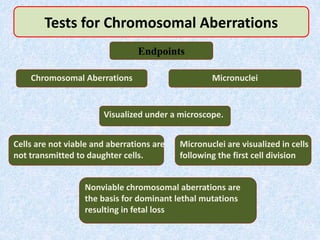
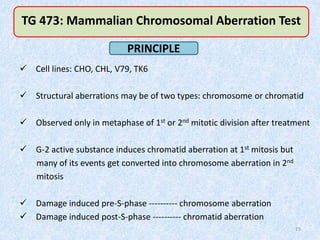
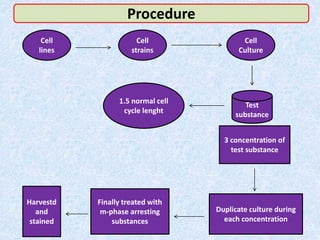
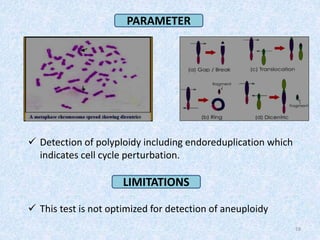
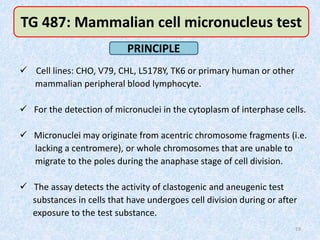
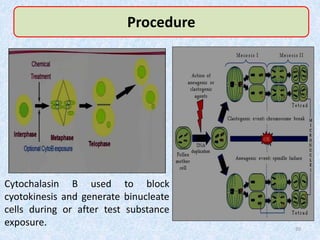
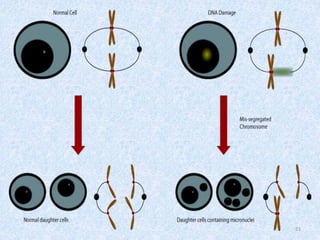
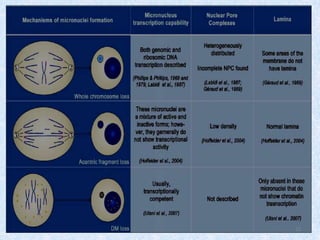
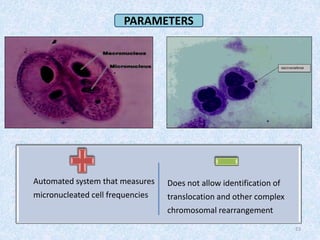
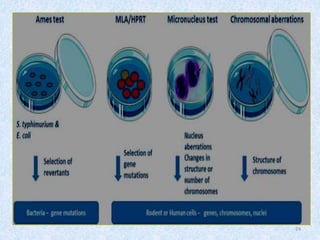
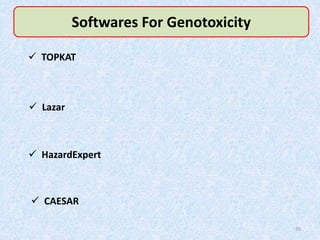
Shital Magar presented on in vitro genotoxicity testing based on OECD guidelines. The presentation covered the objectives of genotoxicity testing, introduction to genotoxicity, history, and details of key in vitro tests including bacterial reverse mutation assay, mammalian cell gene mutation tests, mammalian chromosomal aberration test, and mammalian cell micronucleus test. Parameters and limitations of each test were discussed along with examples of software used to analyze genotoxicity results.