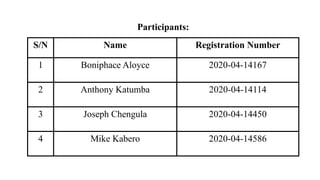
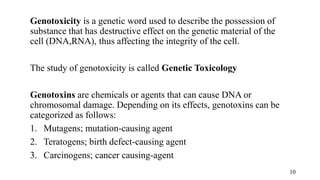
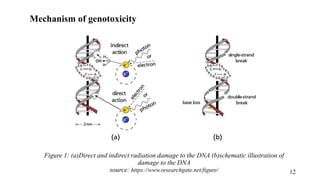
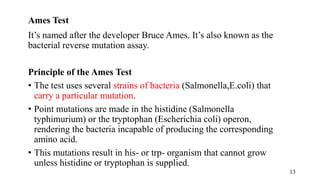
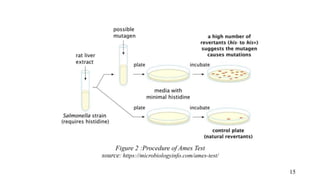
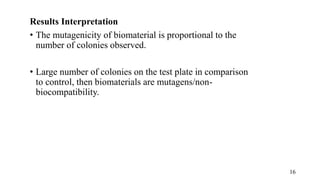
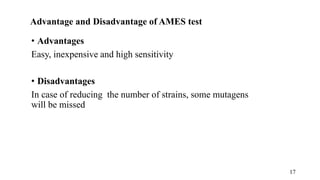
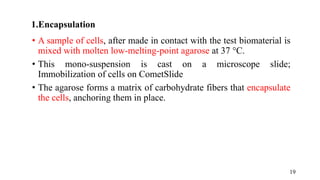
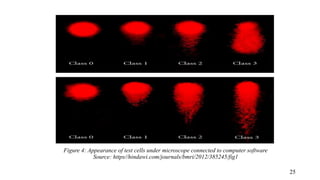
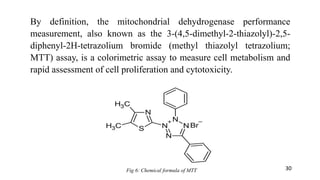
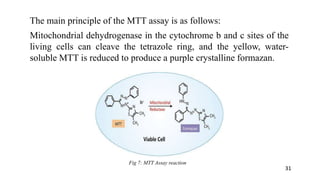
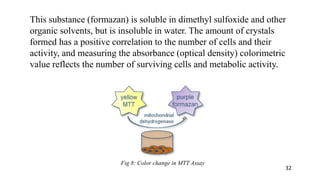
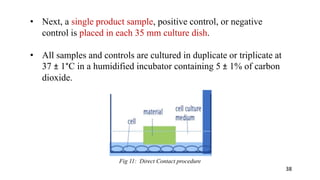
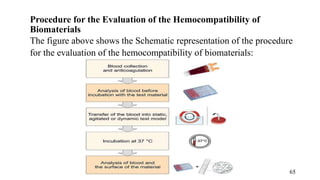
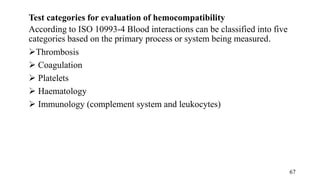
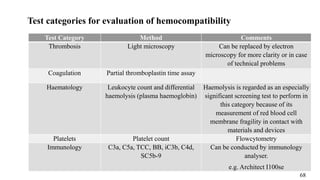
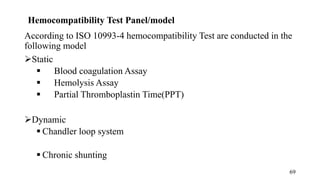
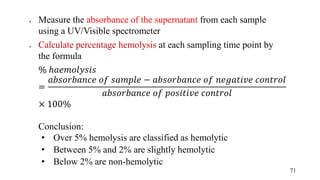
The document provides a comprehensive overview of biocompatibility testing for biomedical engineering, including concepts like cytotoxicity, genotoxicity, immunogenicity, and hemocompatibility. It details various testing methods such as the Ames test, comet test, and cytotoxicity assays, emphasizing the importance of following ISO 10993 standards. Additionally, it categorizes medical devices based on biological effects and highlights the significance of ensuring materials perform safely within the human body.