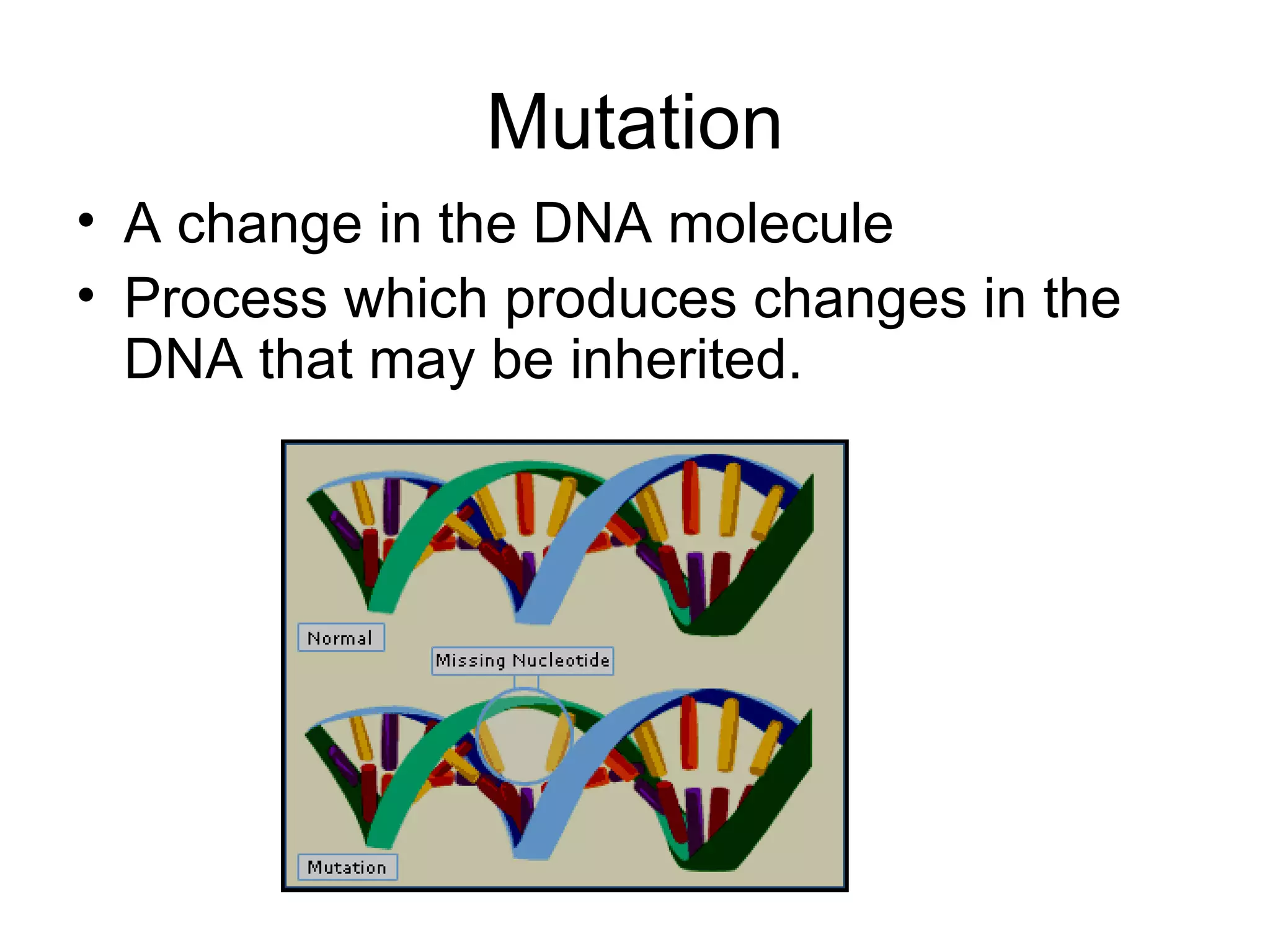
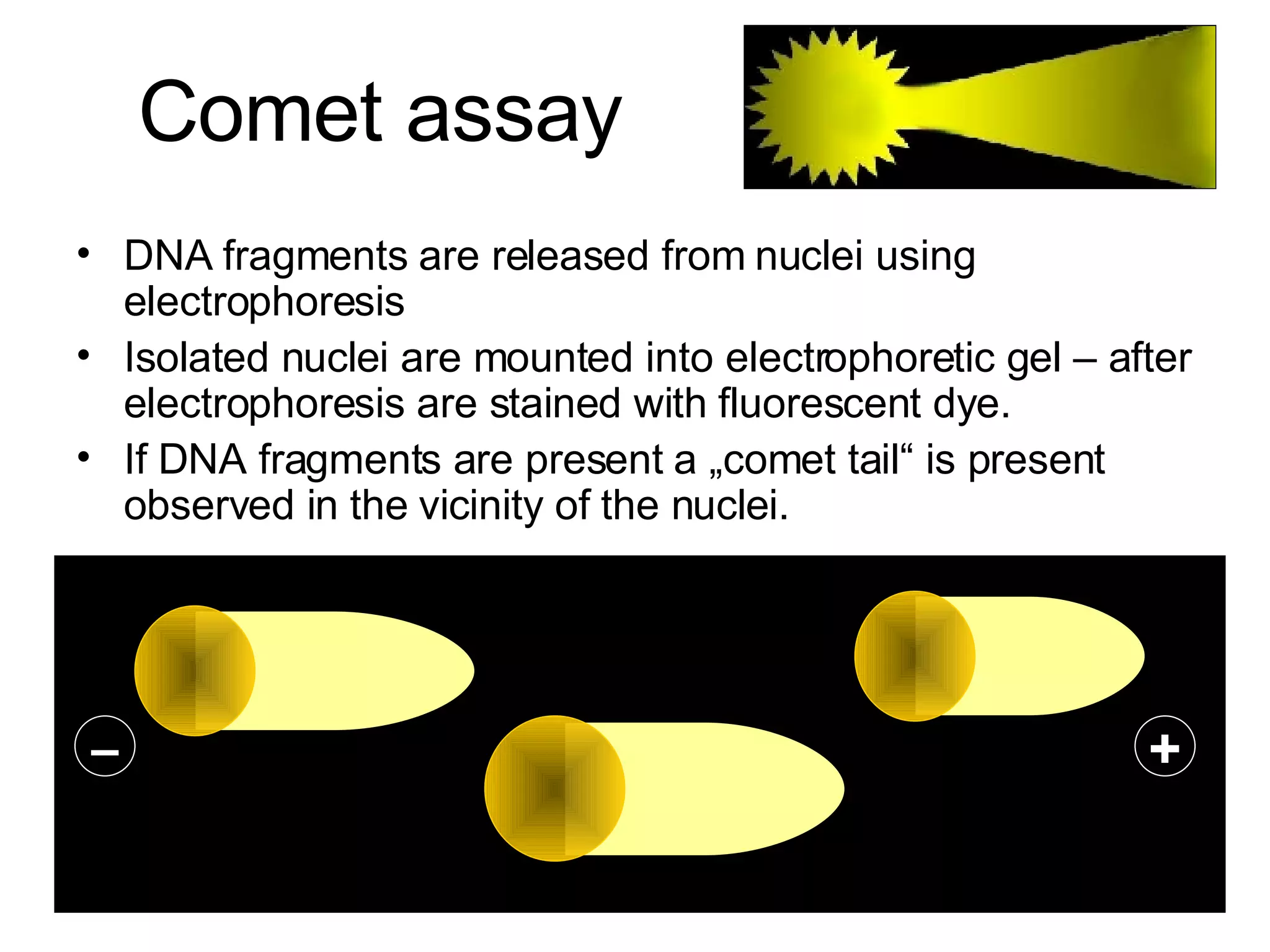
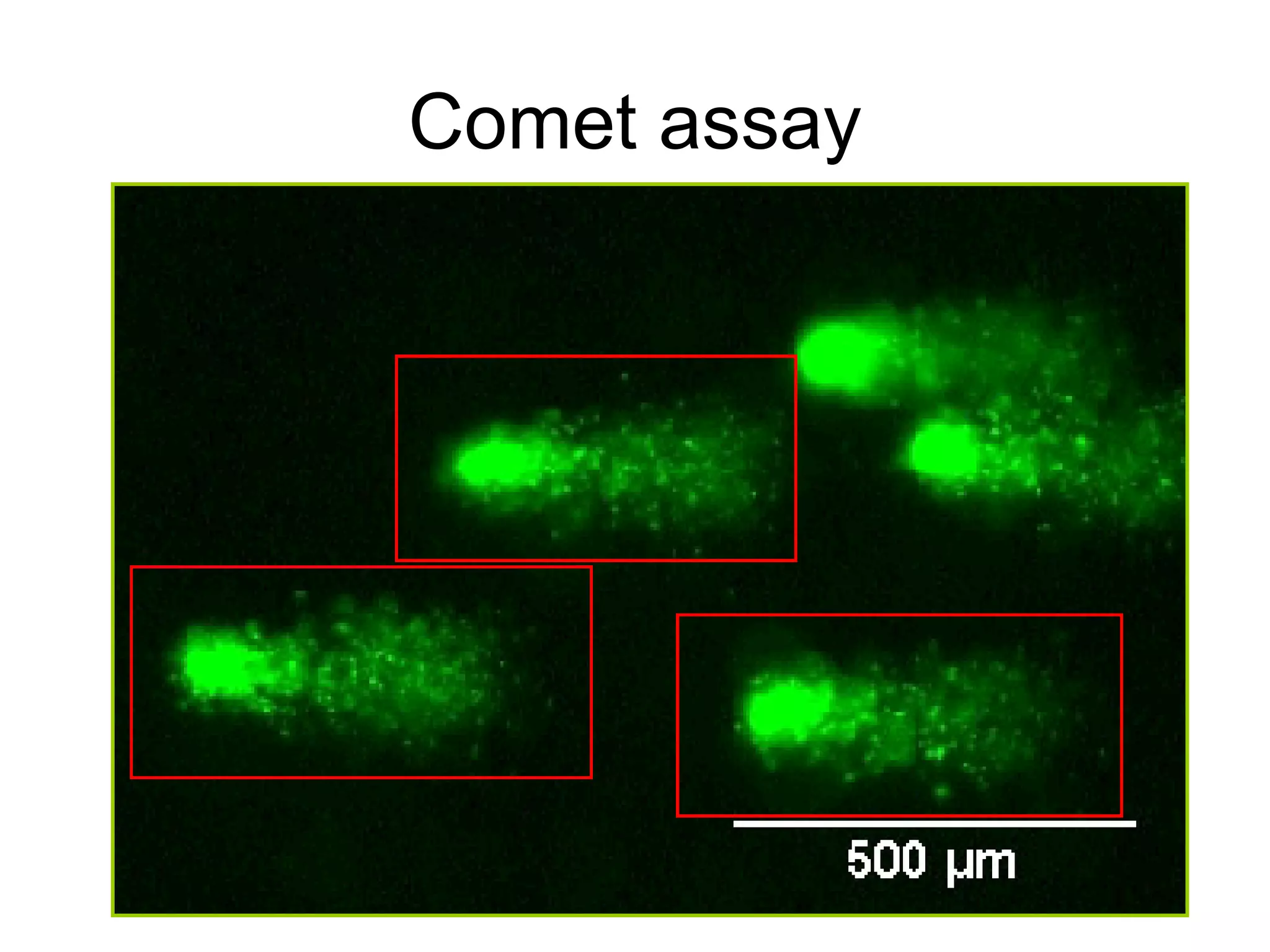
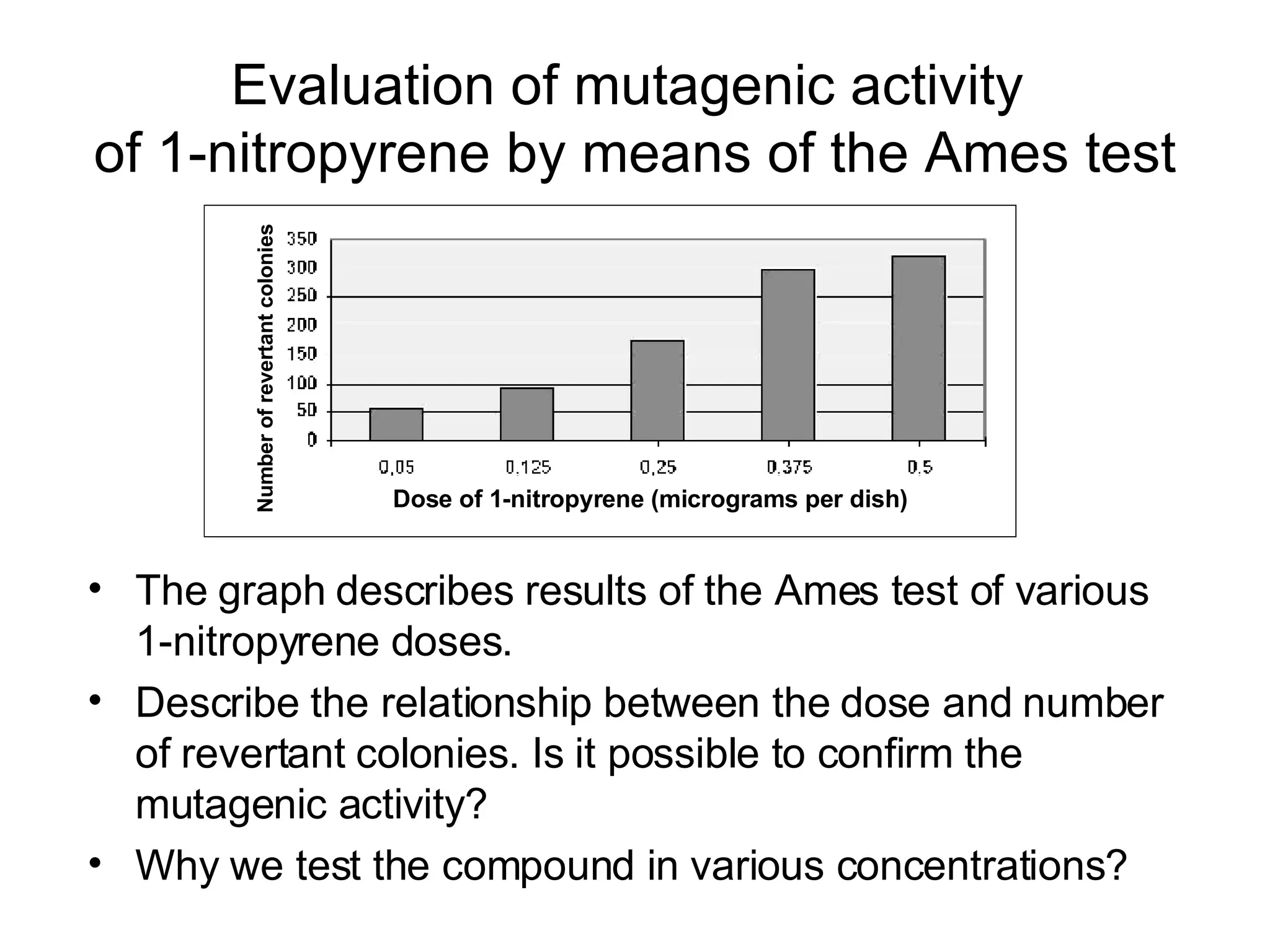
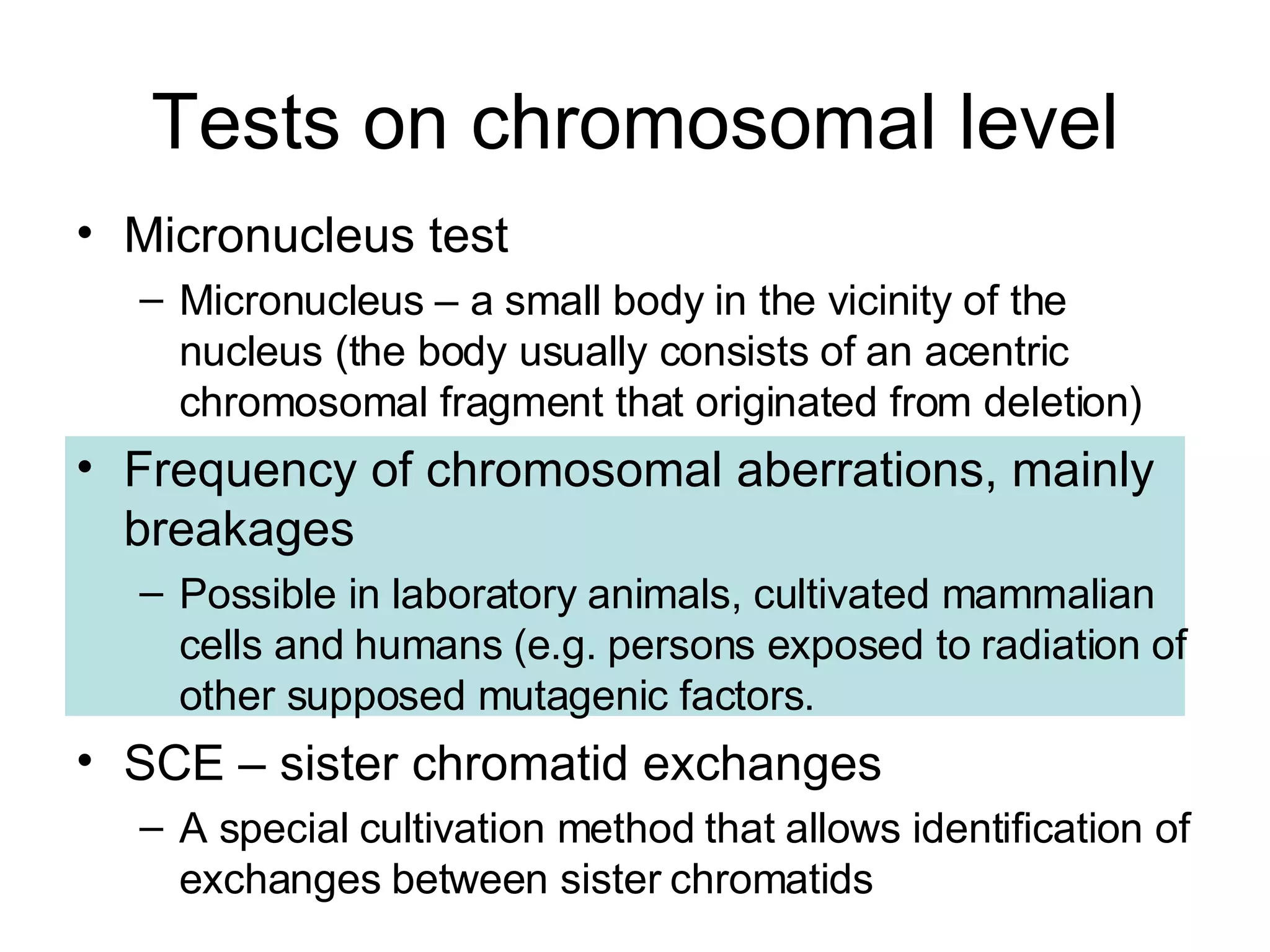
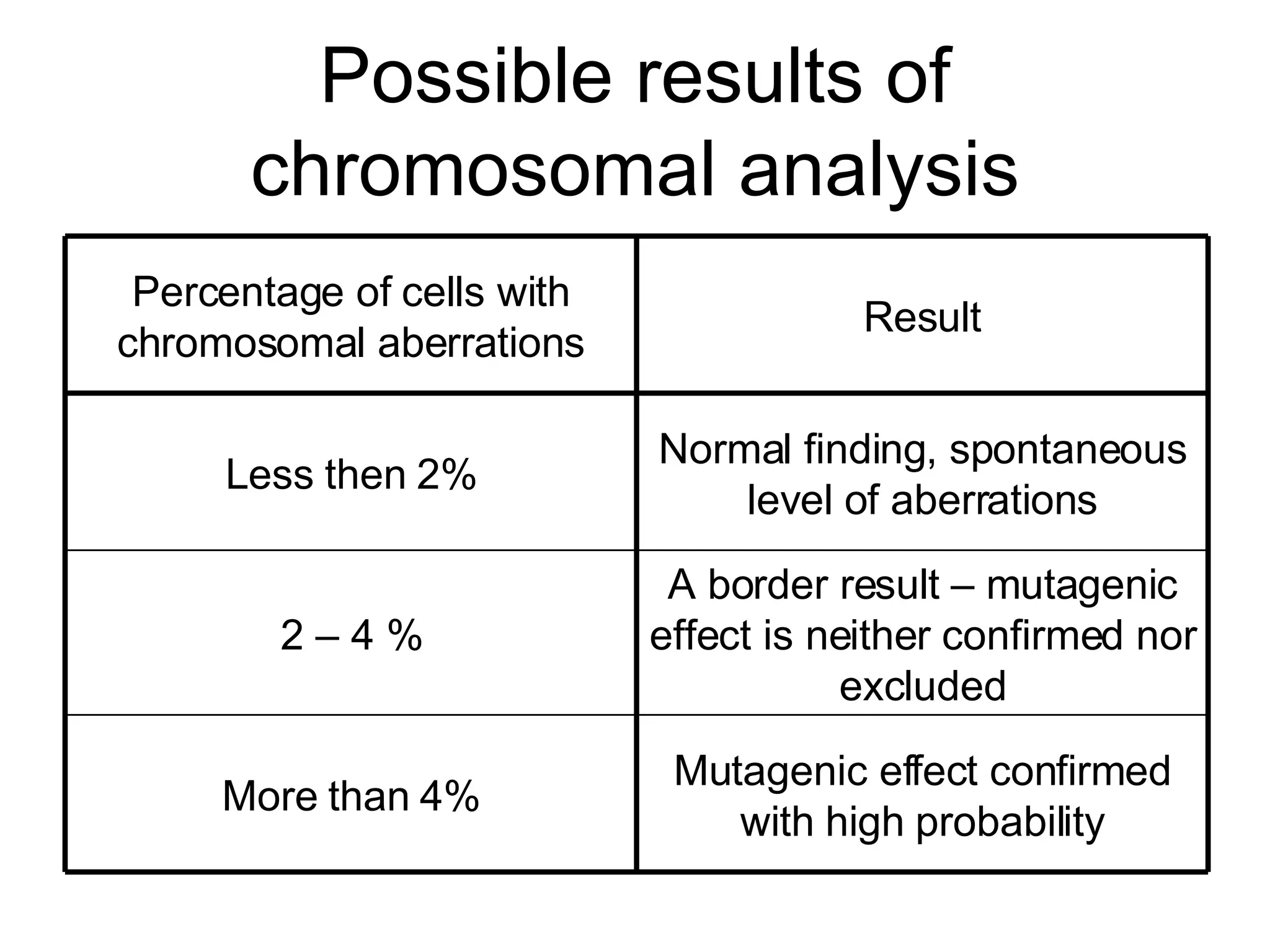
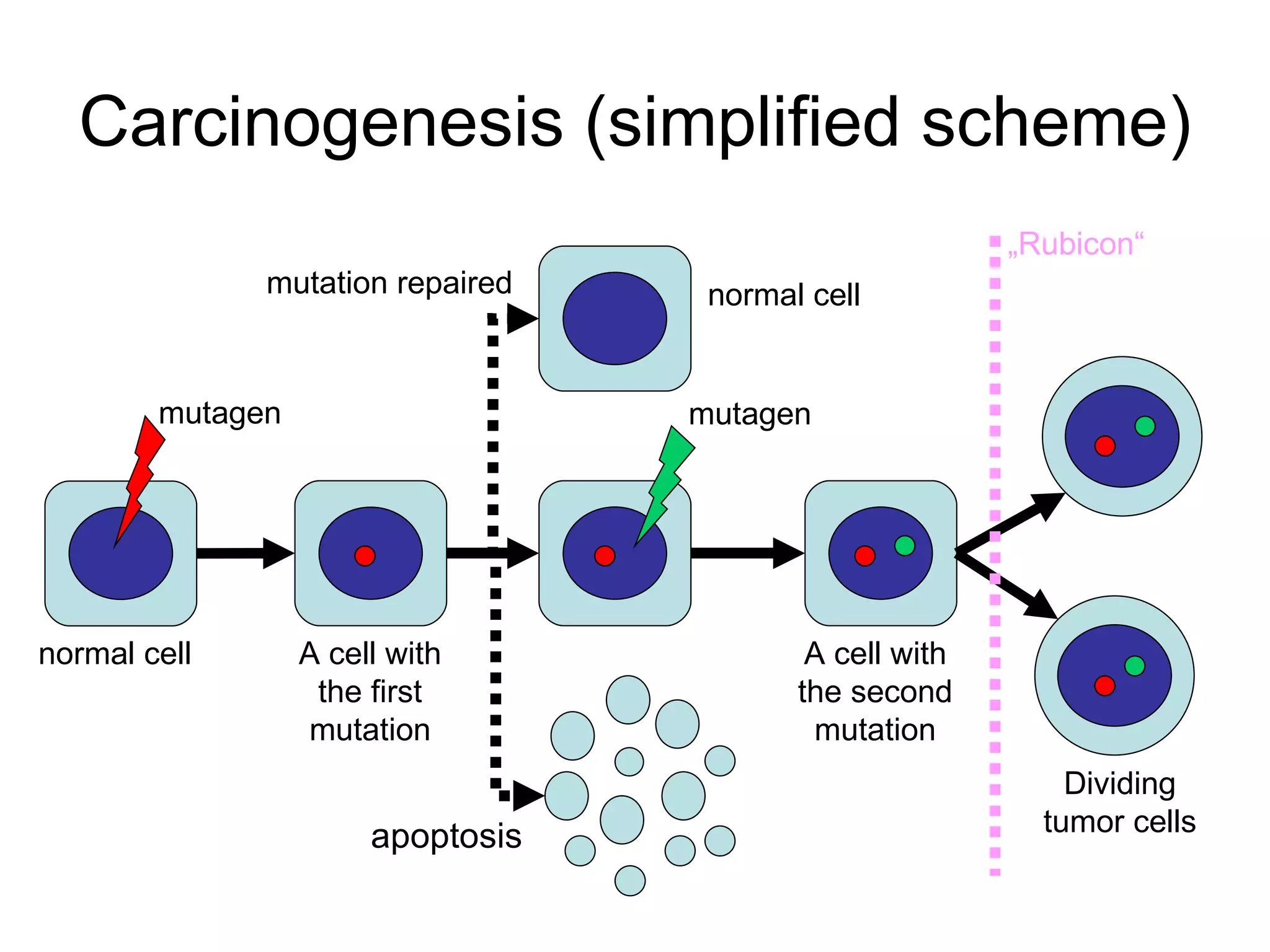
The document discusses mutagenicity and carcinogenicity of environmental factors. It defines key terms like mutagen, mutagenicity, carcinogen and carcinogenicity. It describes various types of mutagens and carcinogens. It also summarizes different methods to test for mutagenicity and carcinogenicity, including tests on the molecular, gene and chromosomal level like the Ames test, comet assay and micronucleus test. The document provides an overview of the process of carcinogenesis and challenges in evaluating human carcinogenicity.