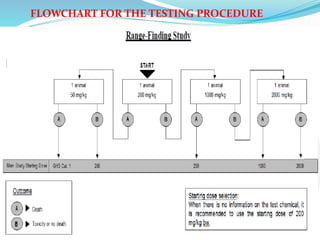
The document outlines the revised OECD test guideline 402 for acute dermal toxicity, emphasizing streamlined testing protocols to reduce the number of animals used while ensuring accuracy in toxicity classification. It details specifications for selecting test animals, administering test items, and observing outcomes, highlighting the need for harmonized classifications based on recent research findings. The document also includes recommendations for the humane treatment of animals and necessary documentation of findings.