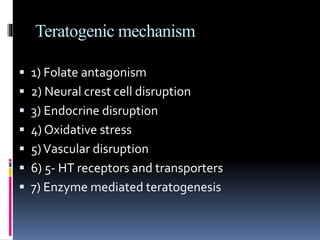
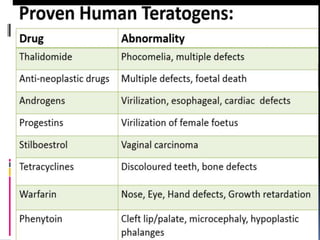
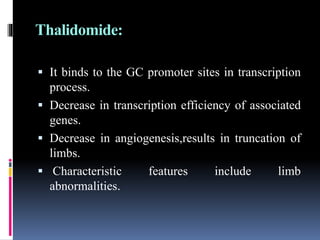
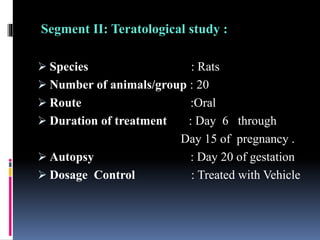
This document discusses teratogenicity studies, which assess the ability of substances to cause birth defects. It covers the mechanisms, principles, types of studies, observations, data reporting, and evaluation. Regarding study types, it describes single generational studies under FDA guidelines to evaluate effects on fertility and reproductive performance. Segment II assesses developmental toxicity by exposing pregnant rats to the test substance from days 6-15 of gestation, then examining fetuses for abnormalities on day 20. The document provides details on study procedures, observations, and reporting of results to evaluate if the substance is teratogenic.