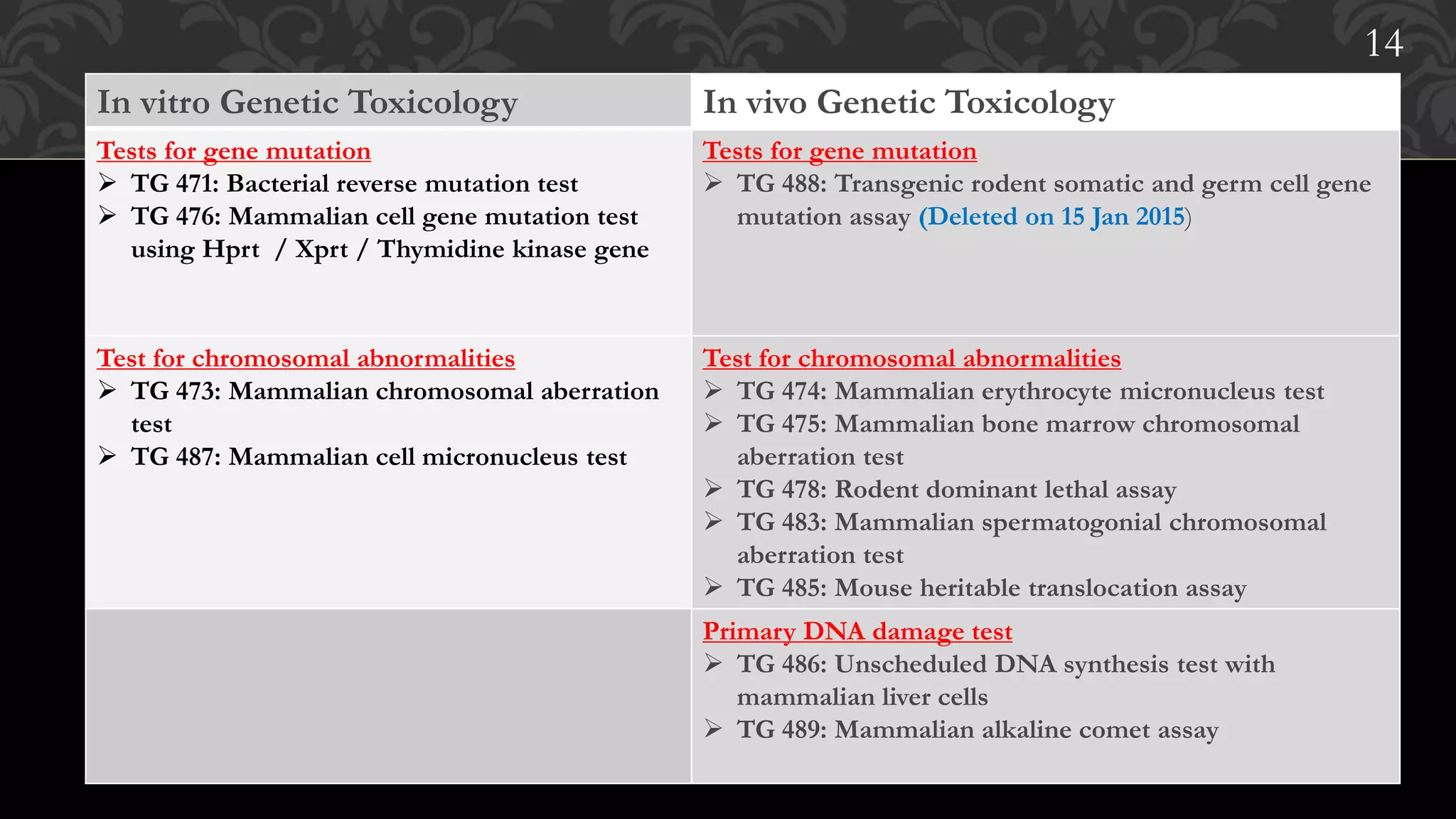
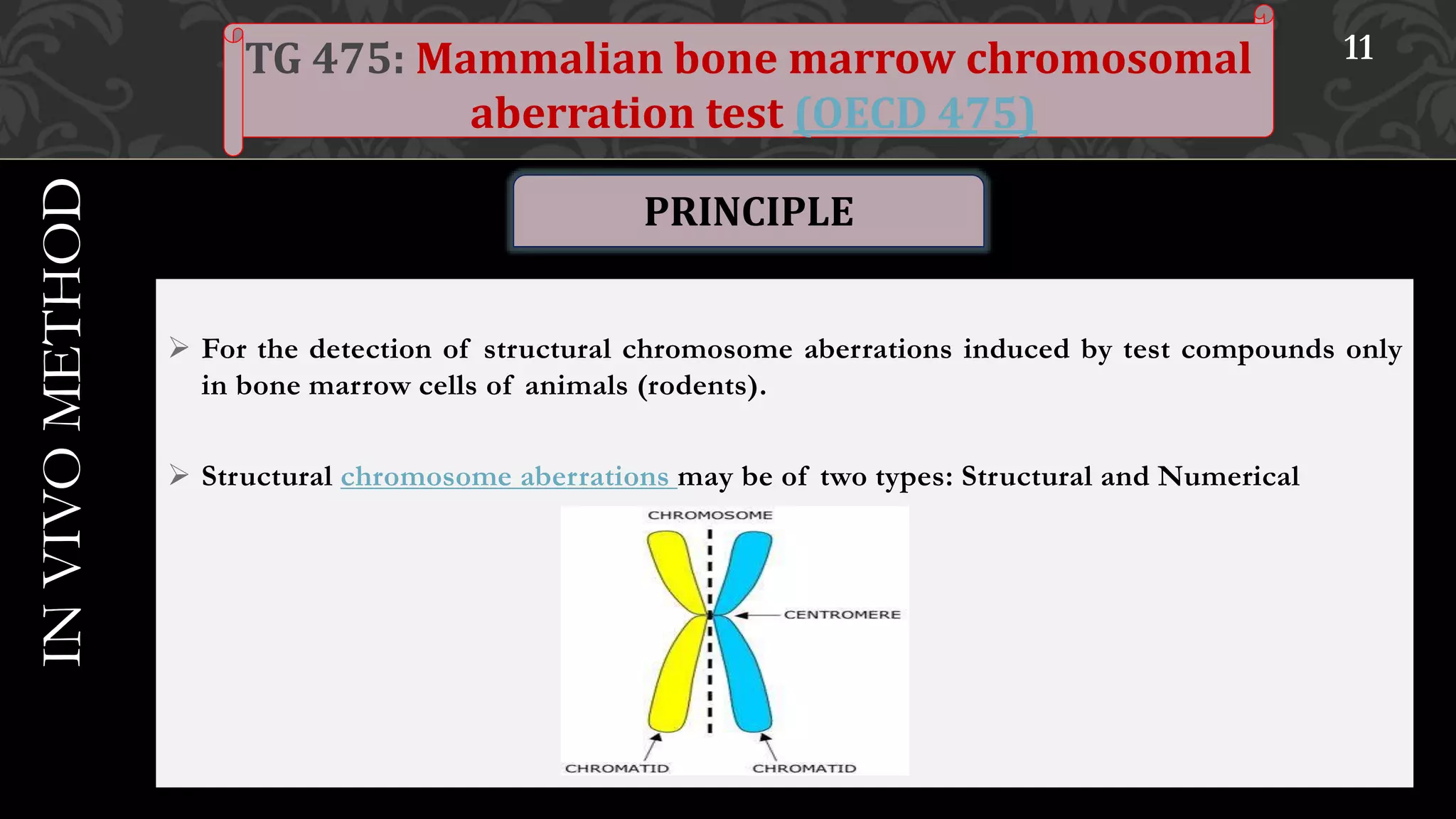
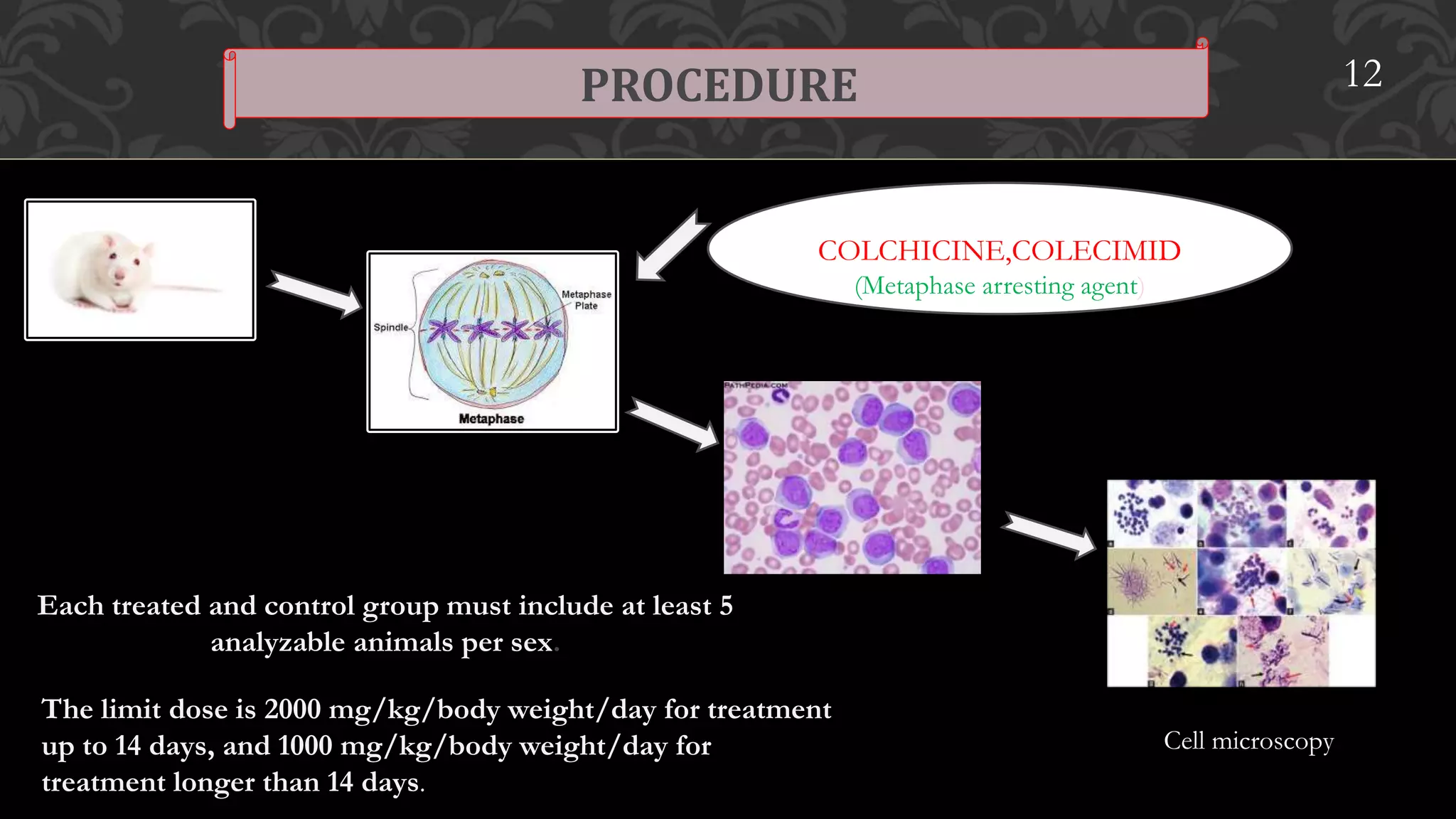
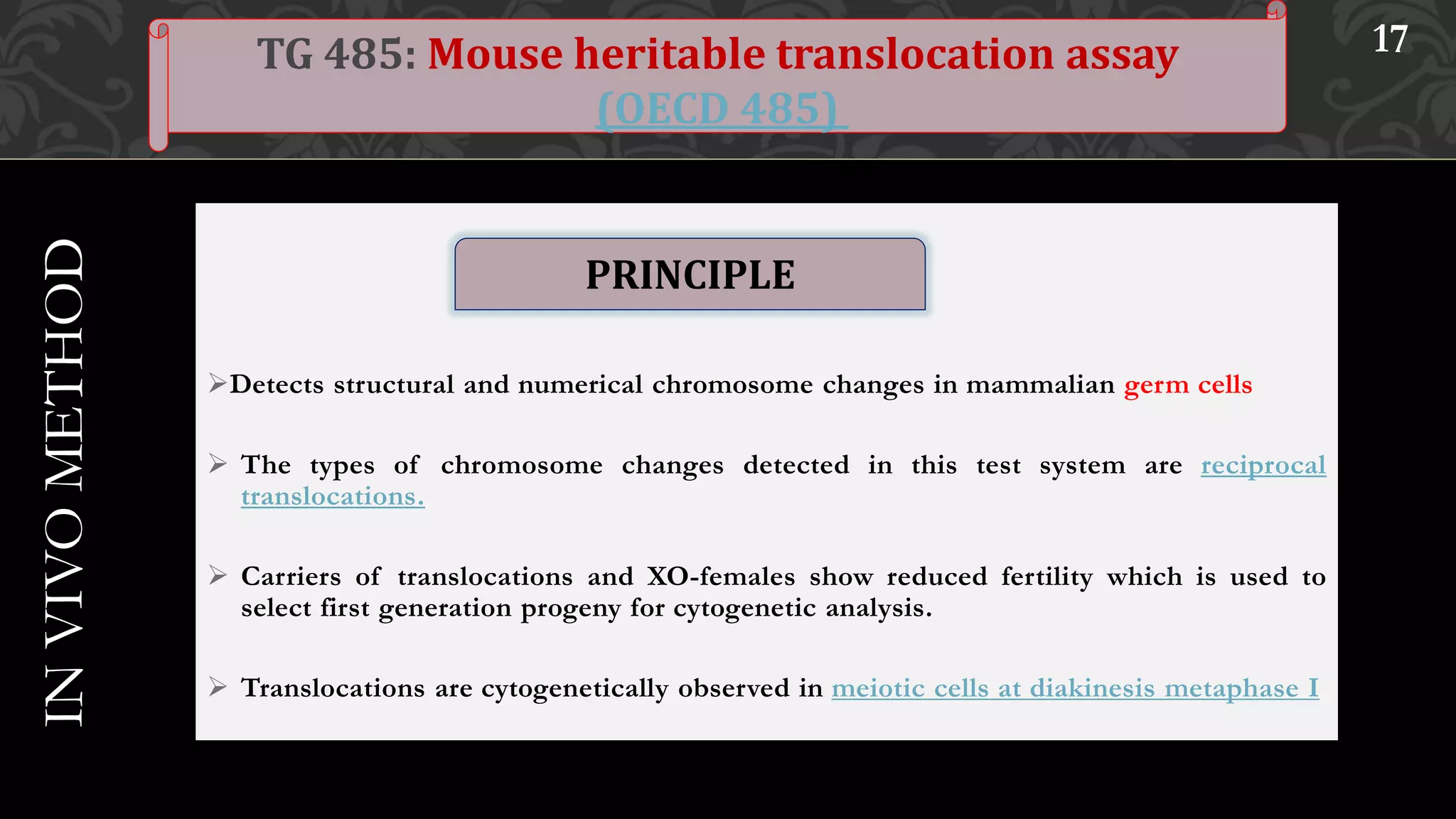
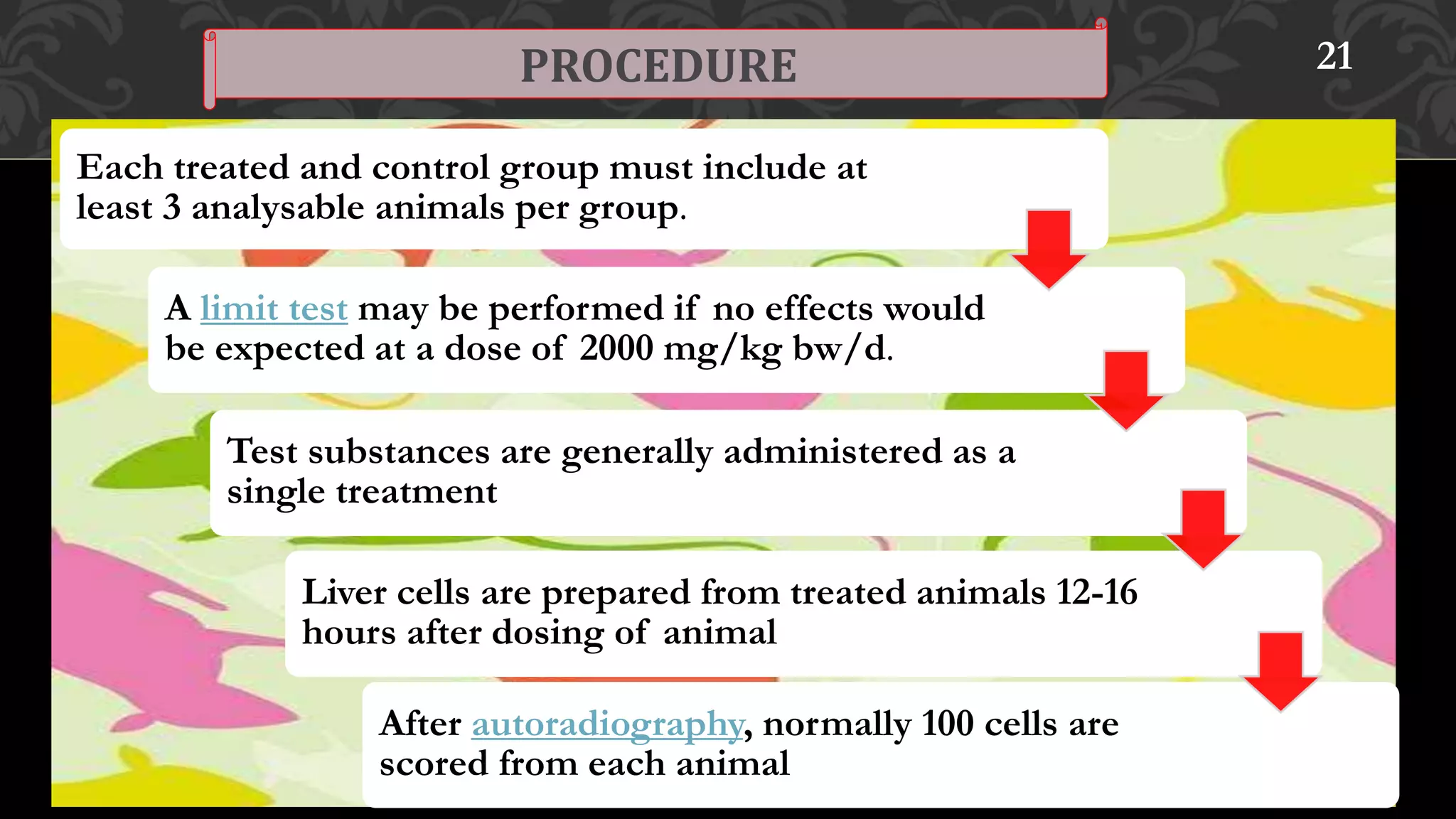
This document provides an overview of genetic toxicity testing guidelines. It discusses the history and aims of toxicity studies. Various in vitro and in vivo genetic toxicology tests are described, including tests for gene mutation, chromosomal abnormalities, and primary DNA damage. Key tests covered include the mammalian erythrocyte micronucleus test, mammalian bone marrow chromosomal aberration test, rodent dominant lethal assay, and mouse heritable translocation assay. The principles, procedures, and parameters of these tests are summarized. References on genetic toxicology guidance documents and studies are also provided.