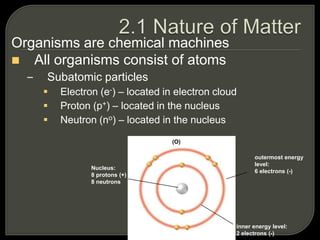
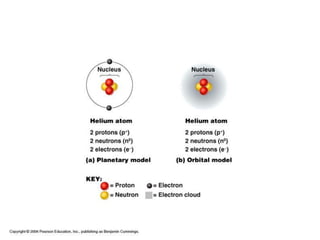
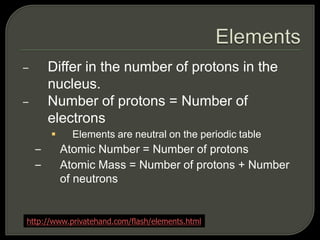
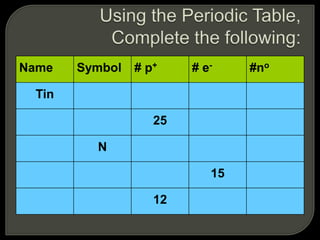
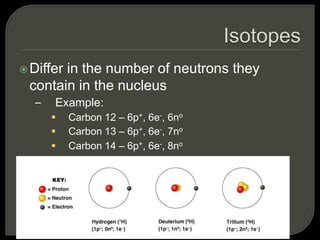
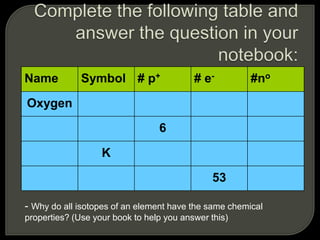
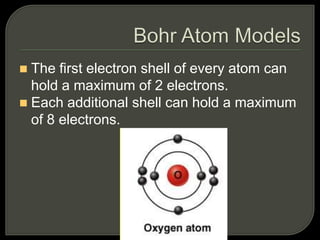
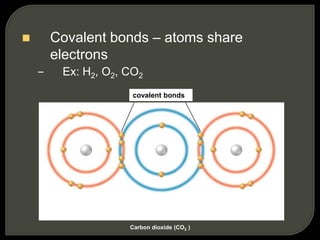
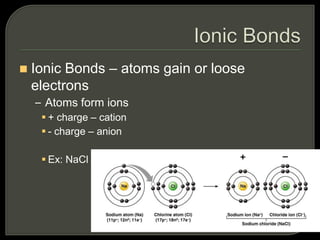
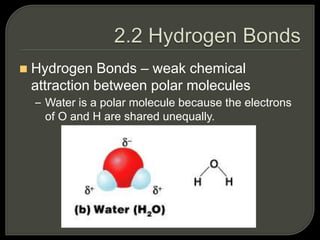
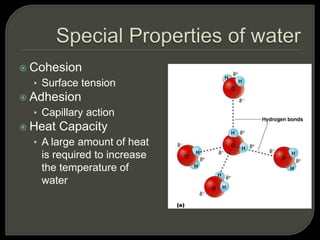
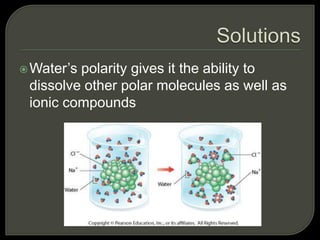
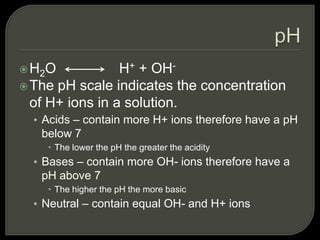
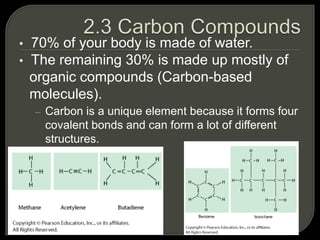
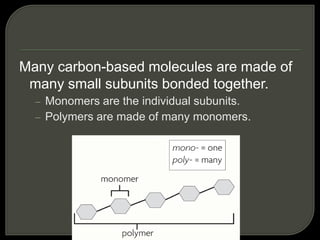
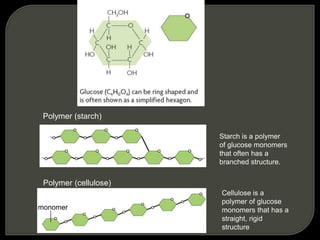
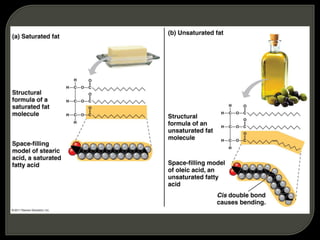
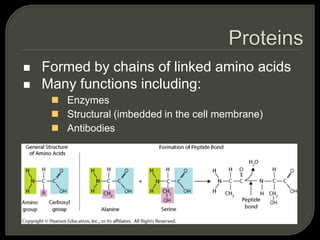
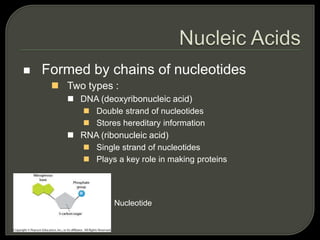
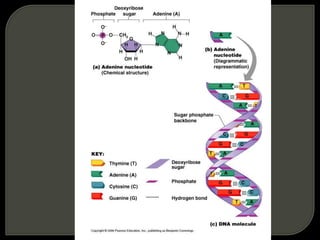
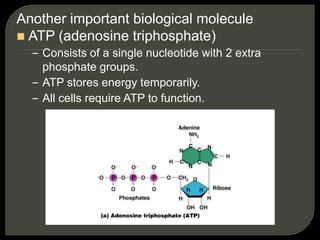
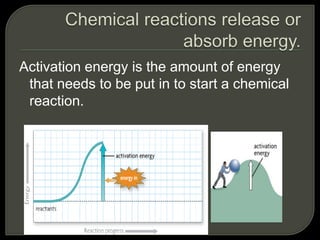
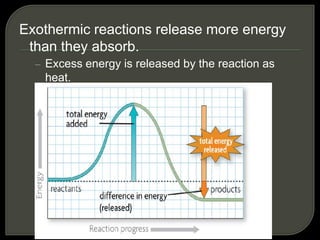
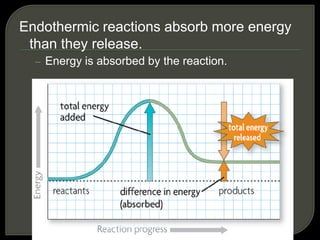
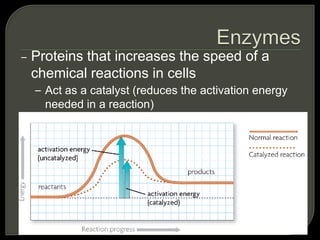
All organisms are made up of chemical compounds composed of atoms. Atoms consist of protons, neutrons, and electrons. Chemical bonds form between atoms, including covalent bonds, ionic bonds, and hydrogen bonds. Important biological molecules include water, carbohydrates, lipids, proteins, nucleic acids, and ATP. These molecules are essential for life and allow organisms to function through various chemical reactions, some of which are catalyzed by protein enzymes.