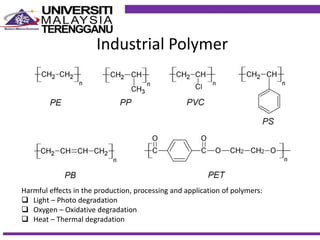
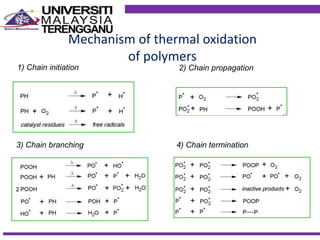
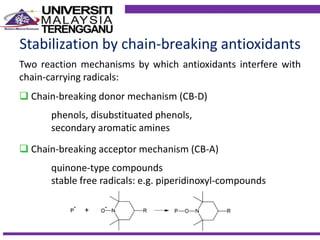
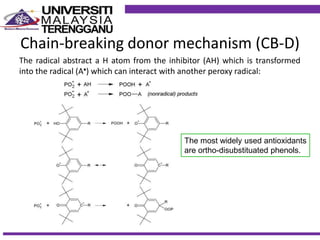
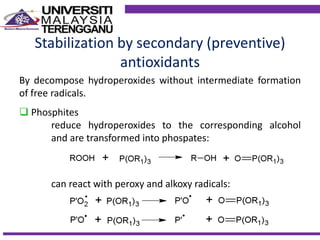
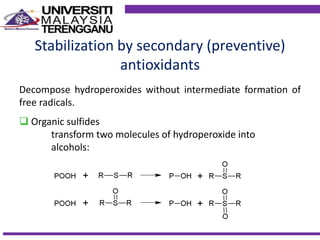
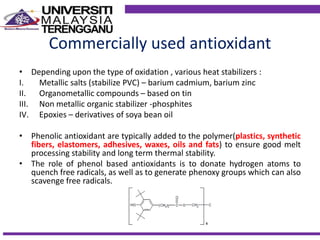
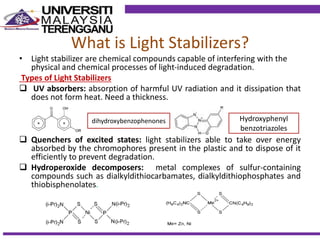
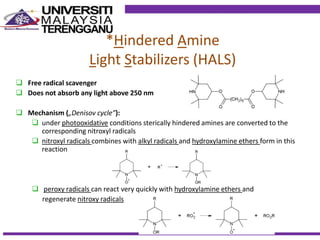
This document discusses stabilizers used in polymers to improve environmental stability against heat, light, and other environmental factors. It defines stabilizers as additives that inhibit polymer degradation and explains their importance. Heat stabilizers discussed include antioxidants that interfere with thermal oxidation through chain-breaking or preventive mechanisms. Light stabilizers described are UV absorbers, quenchers, hydroperoxide decomposers, and hindered amine light stabilizers. The document concludes that stabilizers increase polymer properties like strength and durability but further functionalization can be expensive.