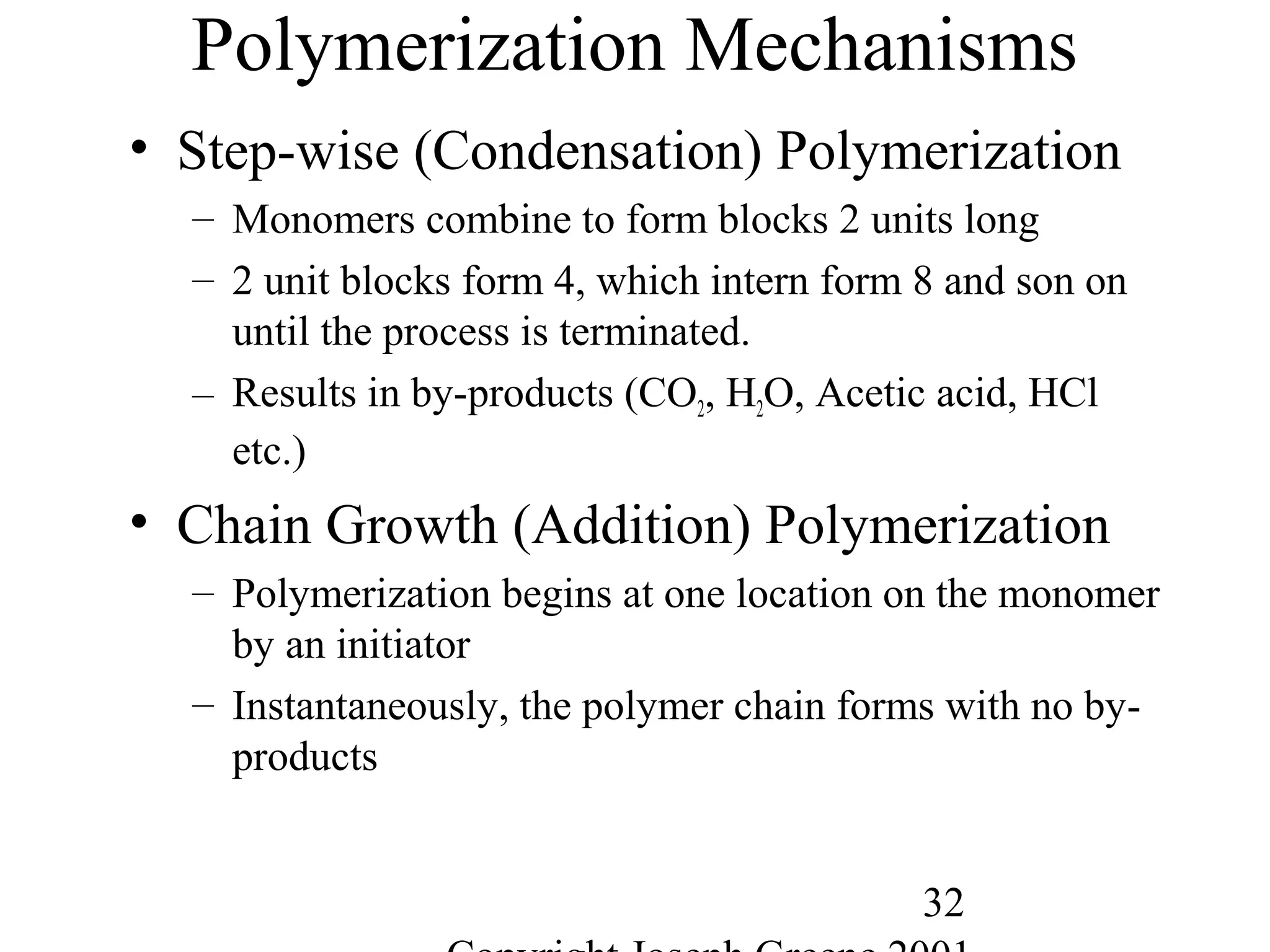
This document provides an overview of polymeric materials called elastomers. It discusses different classes of elastomers including natural rubber, synthetic rubbers, and thermoplastic elastomers. Key points covered include the properties of natural rubber, the vulcanization process, common rubber additives and modifiers, commercial elastomers like styrene-butadiene rubber and their applications. Thermoplastic elastomers are also summarized, focusing on their production methods and advantages over traditional vulcanized rubbers.







![10
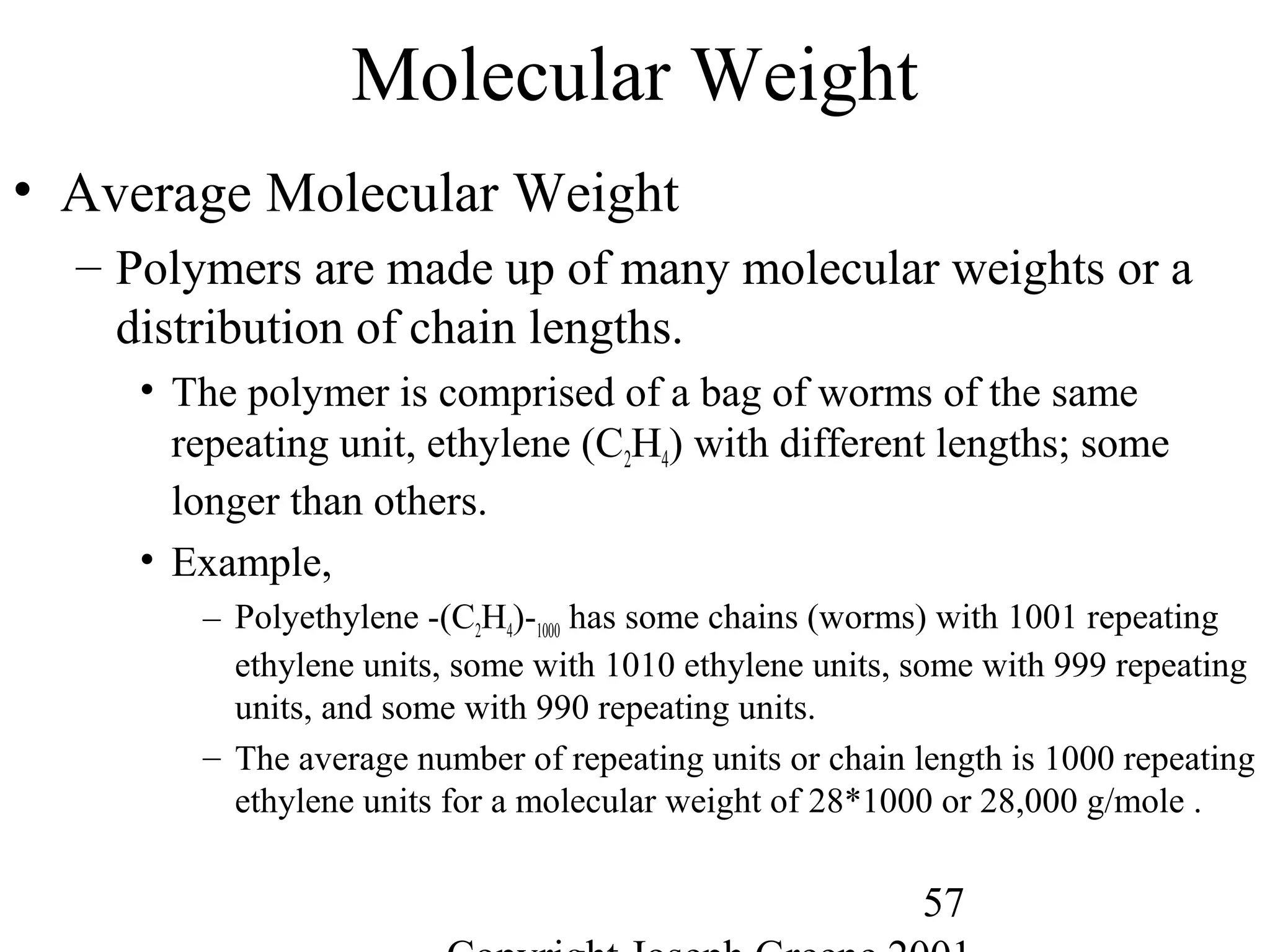
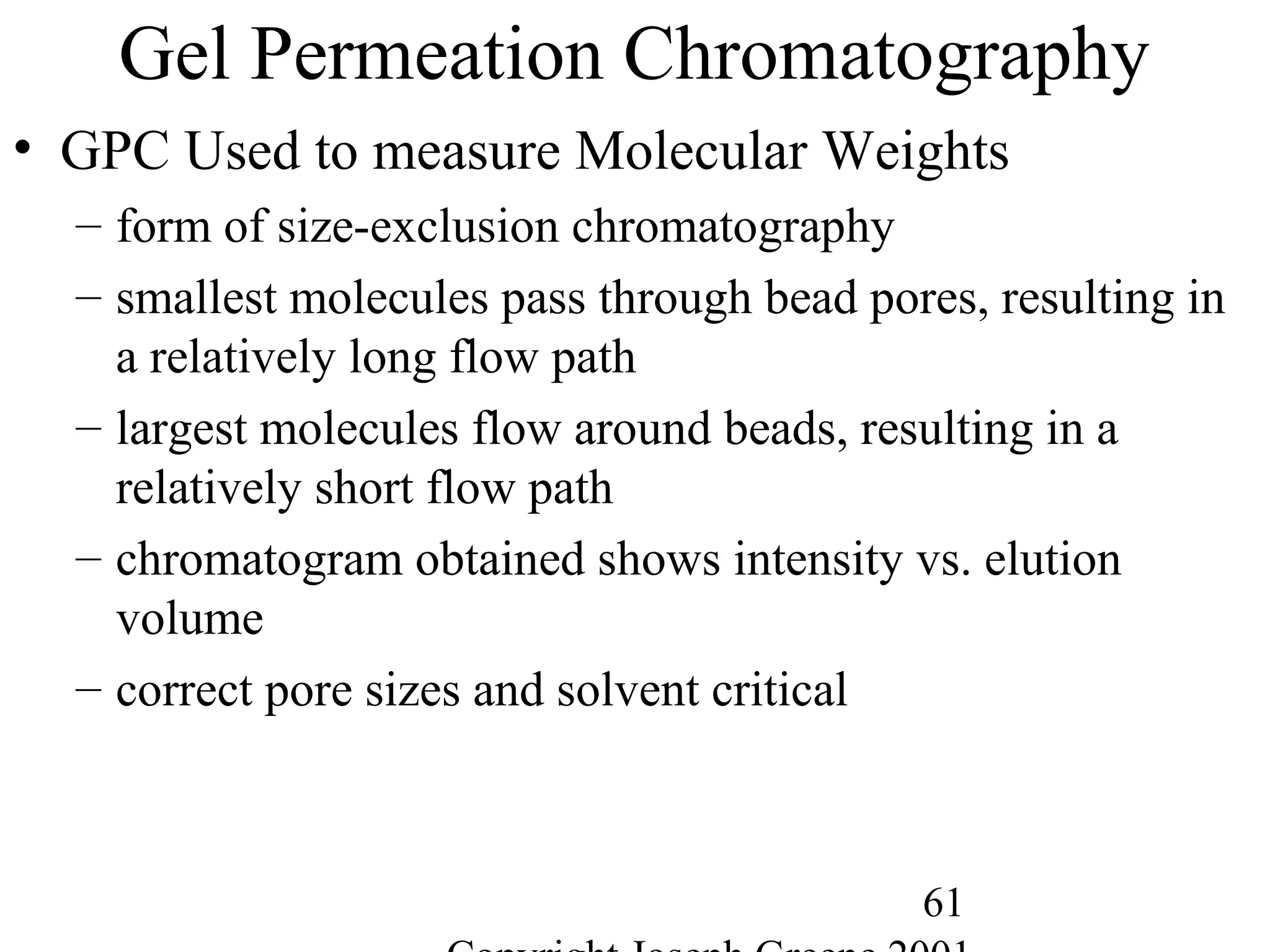
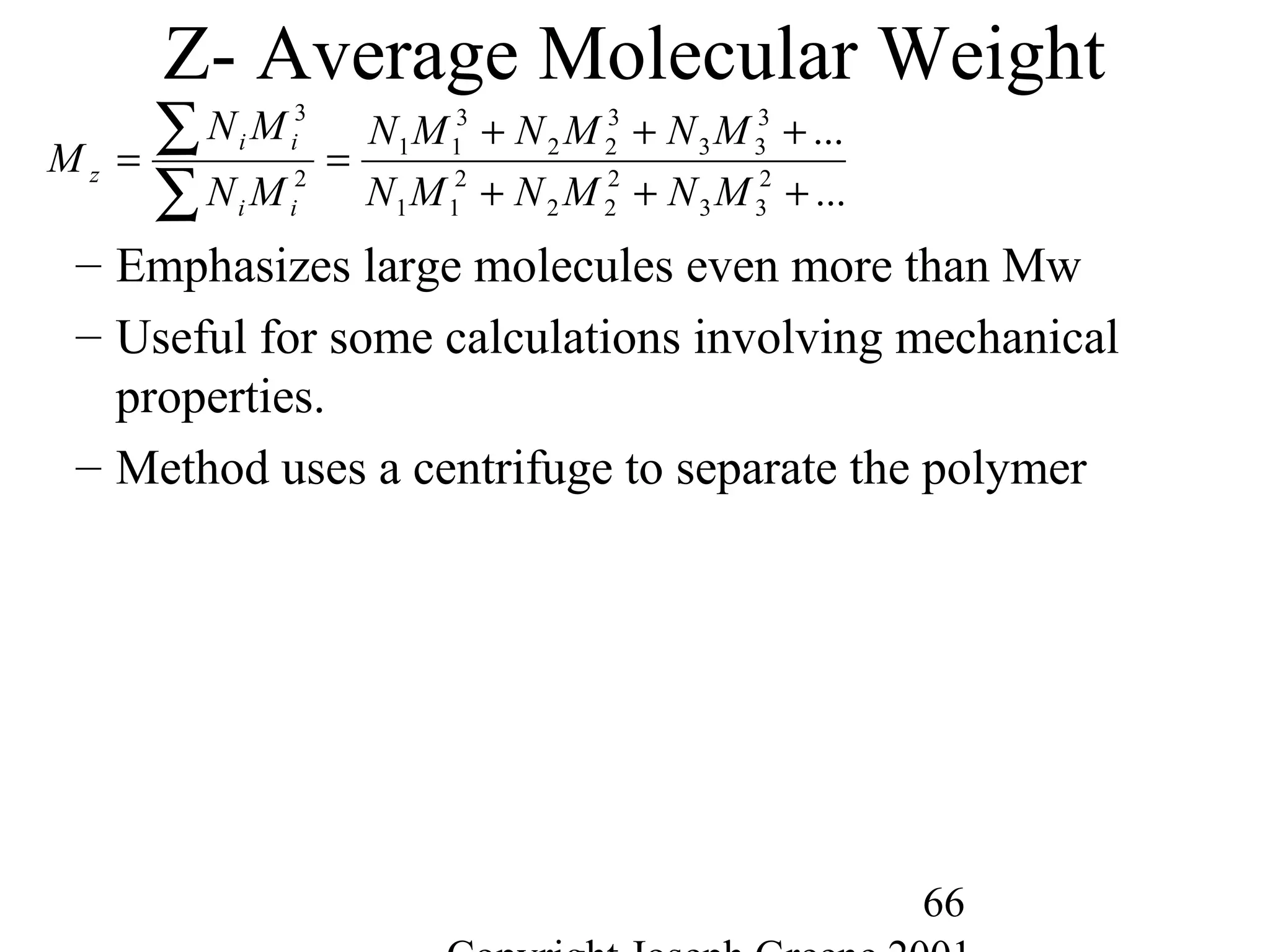
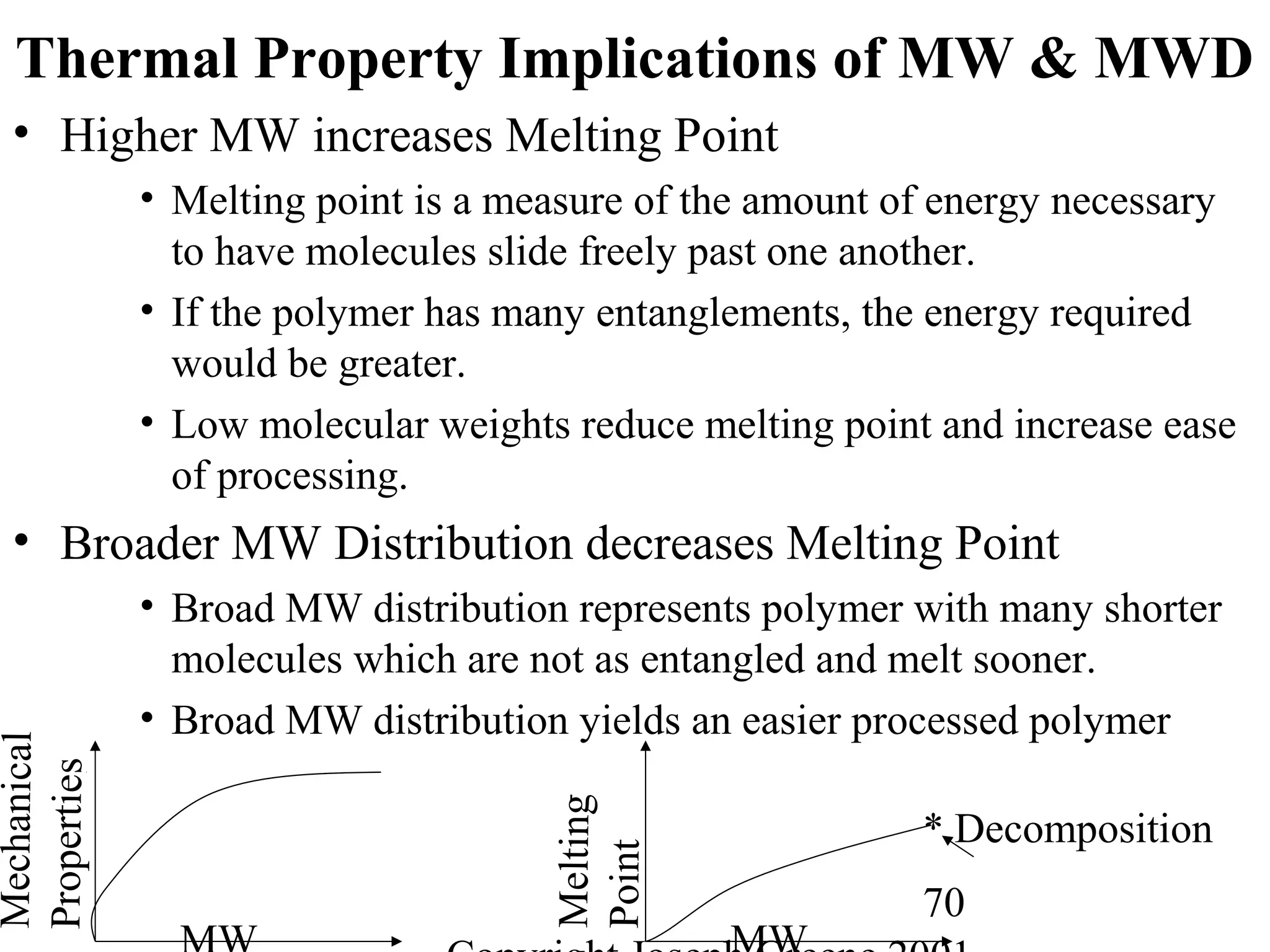
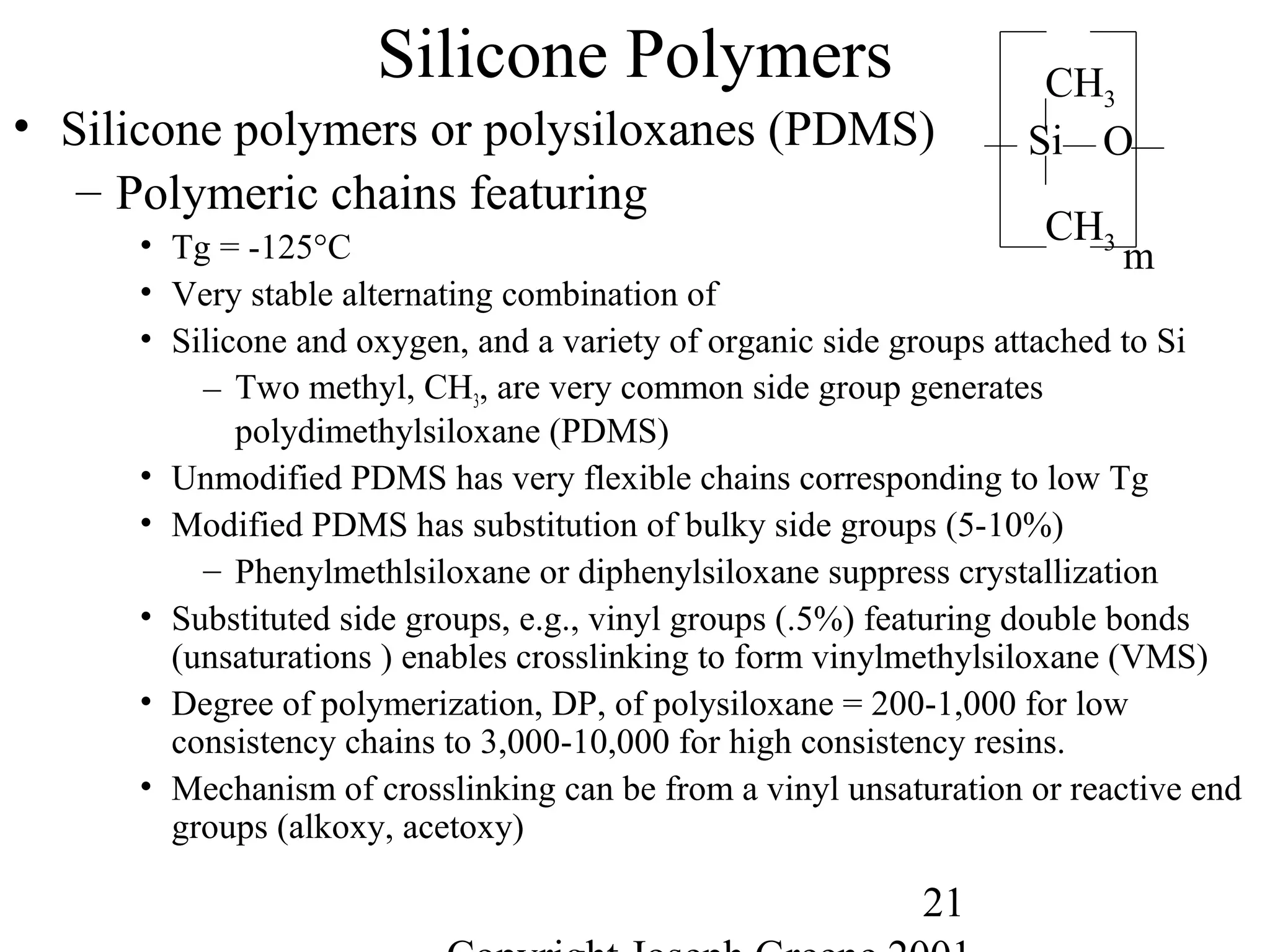
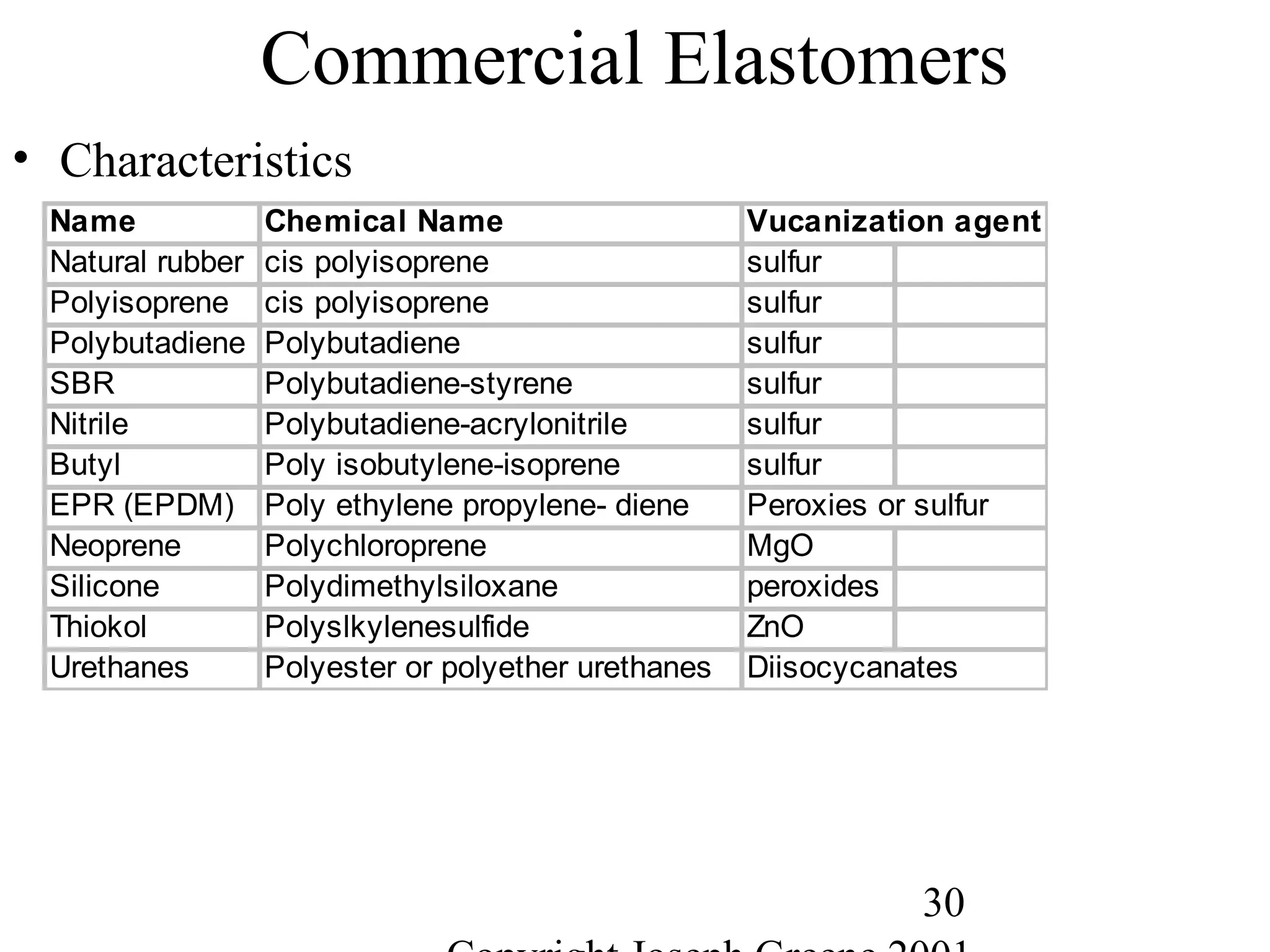
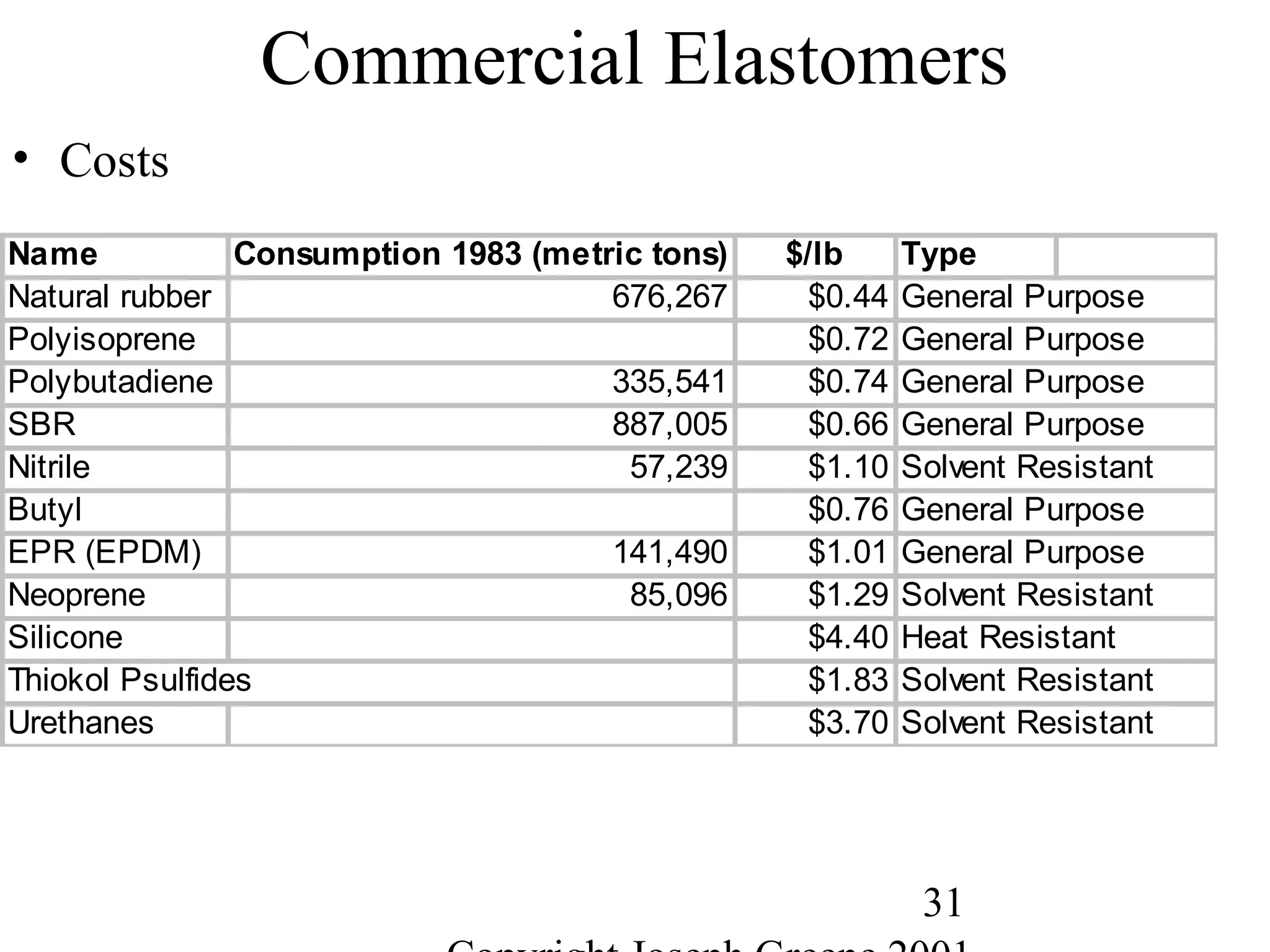
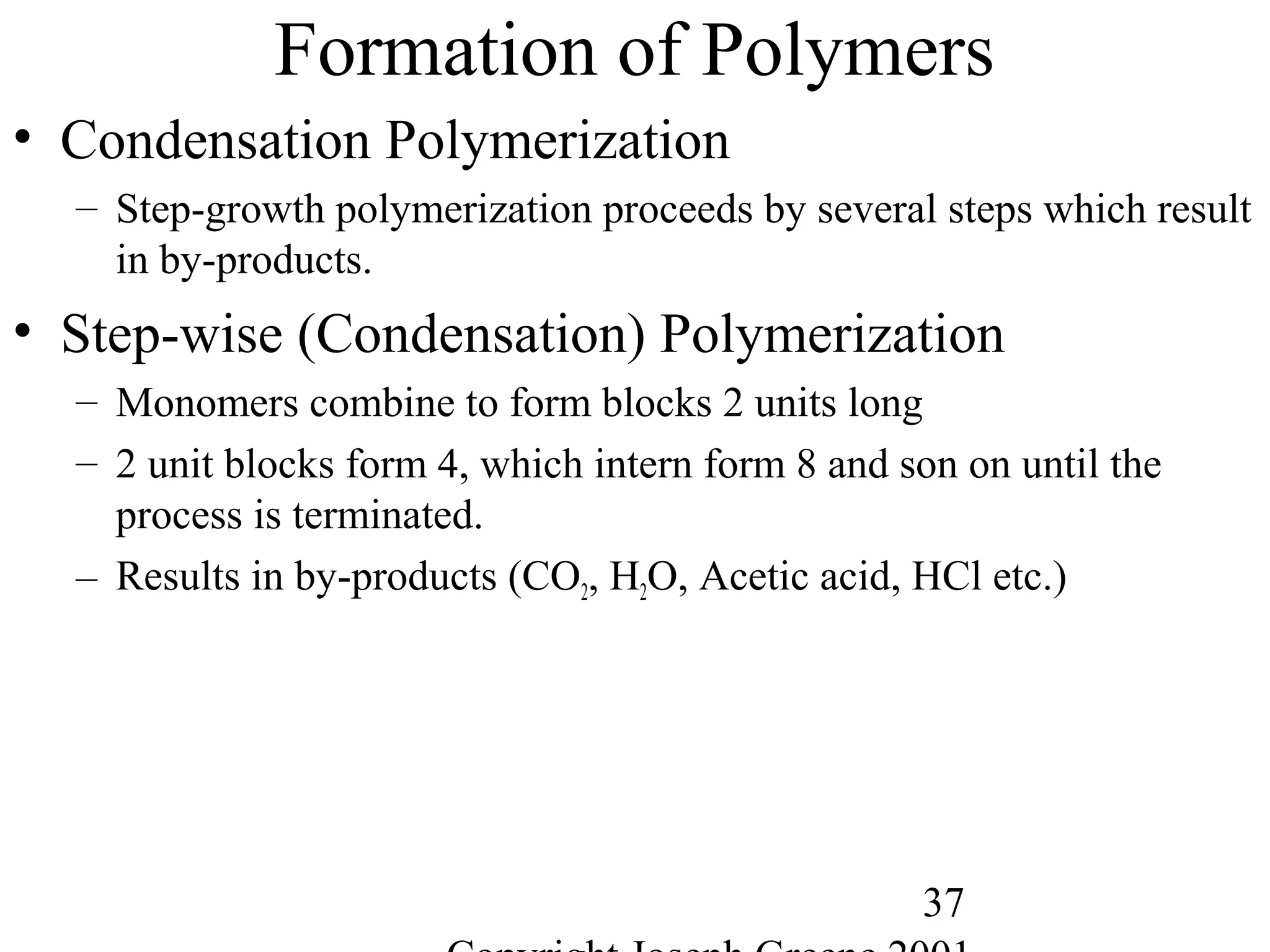
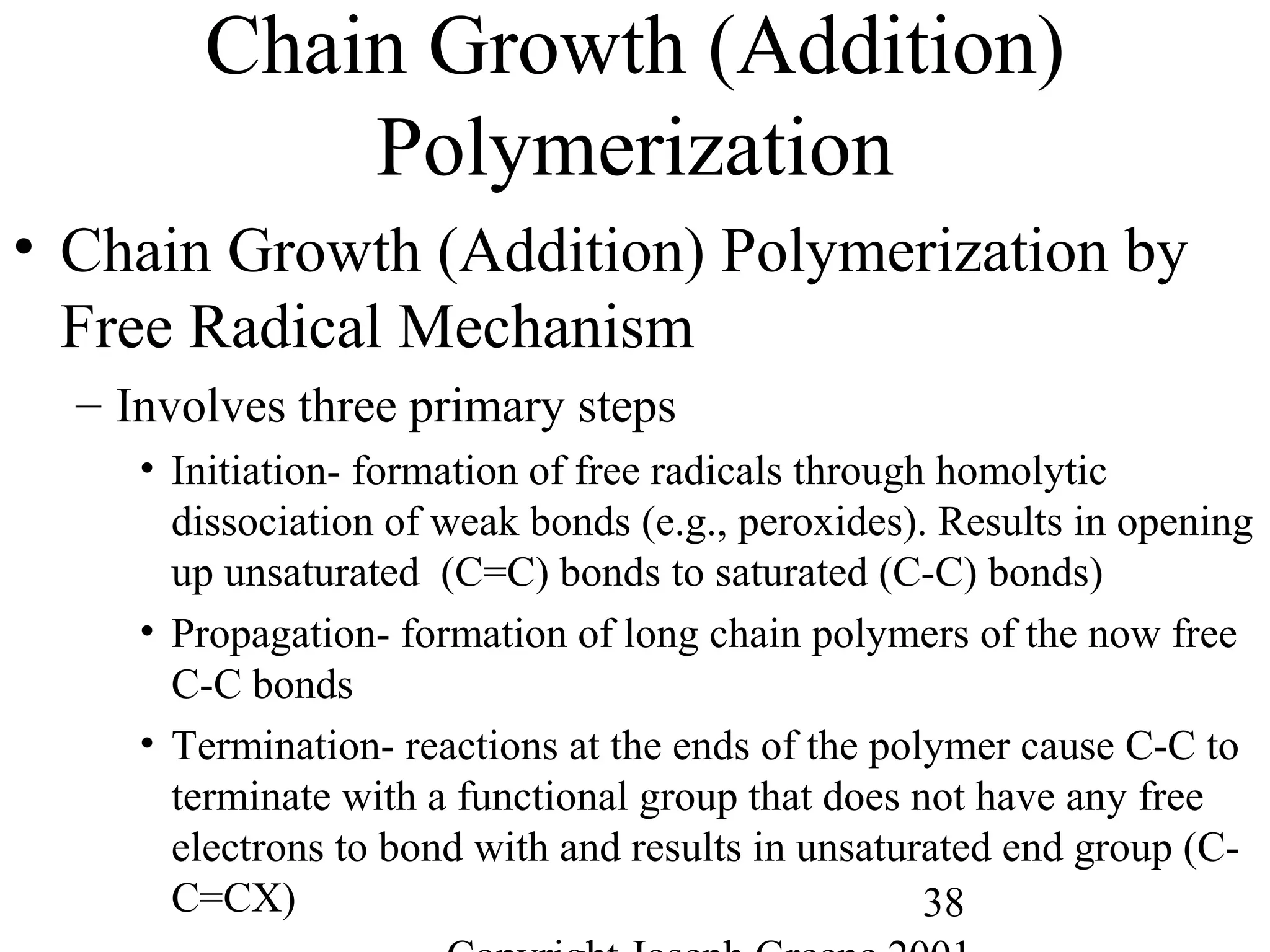
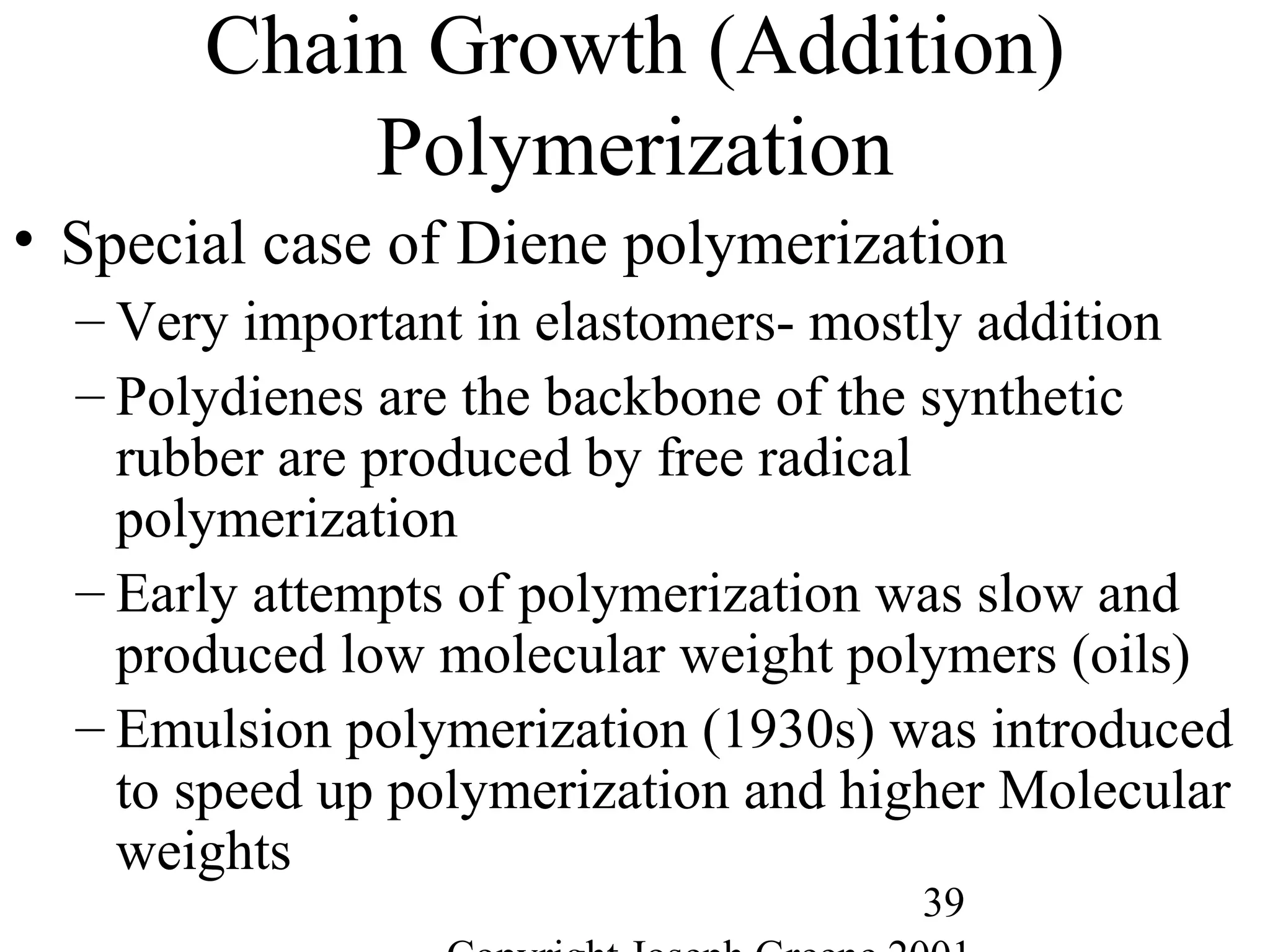
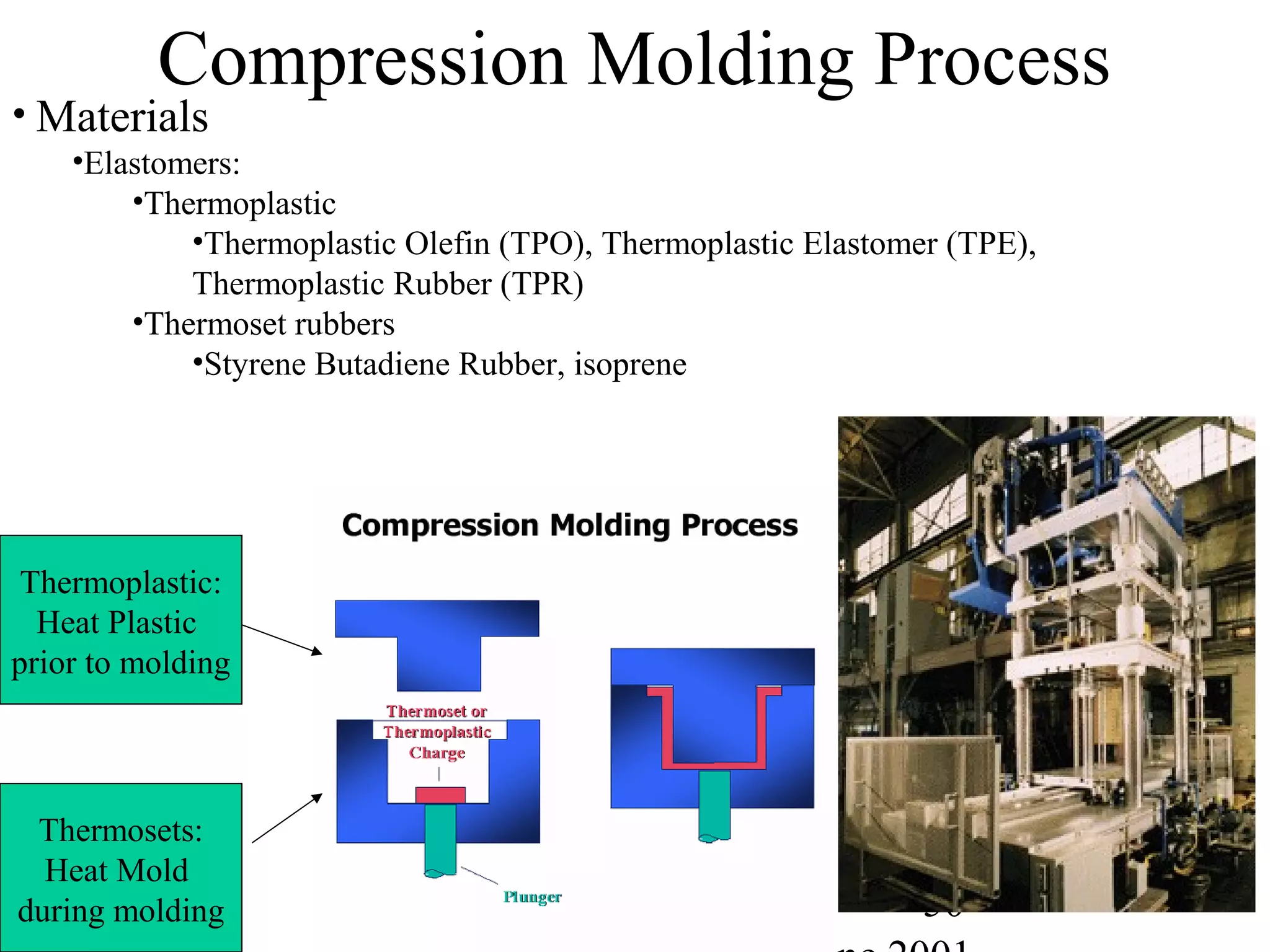
Commercial Elastomers
• Diene C=C double bonds and Related Elastomers
– Polyisoprene- (C5H8)20,000
• Basic structure of natural rubber
• Can be produced as a synthetic polymer
• Capable of very slow crystallization
• Tm = 28°C, Tg = -70°C for cis polyisoprene
• Tm = 68°C, Tg = -70°C for trans polyisoprene
– Trans is major component of gutta percha, the first plastic
– Natural rubber was first crosslinked into highly elastic network by
Charles Goodyear (vulcanization with sulfur in 1837)
• Sulfur crosslinked with the unsaturations C=C
– Natural rubber in unfilled form is widely used for products with
• very large elastic deformations or very high resilience,
• resistance to cold flow (low compression set) and
• resistance to abrasion, wear, and fatigue.
– Natural rubber does not have good intrinsic resistance to sunlight,
oxygen, ozone, heat aging, oils, or fuels.
C
H
C
C
H
H3HH
H
C C ][
C
H
C
H
HH
H
C C ][
CH3
Cis
Trans](https://image.slidesharecdn.com/m246intro-elast-190727082105/75/ABOUT-ELASTOMER-TYPES-AND-VULCANISATION-10-2048.jpg)
![11
Commercial Elastomers
• Polybutadiene
– Basis for synthetic rubber as a major component in copolymers
Styrene-Butadiene Rubber (SBR, NBR) or in
– Blends with other rubbers (NR, SBR)
– Can improve low-temperature properties, resilence, and abrasion
or wear resistance
• Tg = -50°C
• Polychloroprene
– Polychloroprene or neoprene was the very first synthetic rubber
– Due to polar nature of molecule from Cl atom it has very good
resistance to oils and is flame resistant (Cl gas coats surface)
– Used for fuel lines, hoses, gaskets, cable covers, protective boots,
bridge pads, roofing materials, fabric coatings, and adhesives
– Tg = -65°C.
H H
C
H
C
H HH
C C ][
H H
C
H
C
Cl HH
C C ][](https://image.slidesharecdn.com/m246intro-elast-190727082105/75/ABOUT-ELASTOMER-TYPES-AND-VULCANISATION-11-2048.jpg)
![12
Commercial Elastomers
• Butyl rubber- addition polymer of isobutylene.
– Copolymer with a few isoprene units, Tg =-65°C
– Contains only a few percent double bonds from isoprene
– Small extent of saturation are used for vulcanization
– Good regularity of the polymer chain makes it possible for the
elastomer to crystallize on stretching
– Soft polymer is usually compounded with carbon black to increase
modulus
• Nitrile rubber
– Copolymer of butadiene and acrylonitrile
– Solvent resistant rubber due to nitrile C:::N
– Irregular chain structure will not crystallize on stretching, like
SBR
– vulcanization is achieved with sulfur like SBR and natural rubber
• Thiokol- ethylene dichloride polymerized with sodium
H H3
C
CH3H
C
C
][](https://image.slidesharecdn.com/m246intro-elast-190727082105/75/ABOUT-ELASTOMER-TYPES-AND-VULCANISATION-12-2048.jpg)

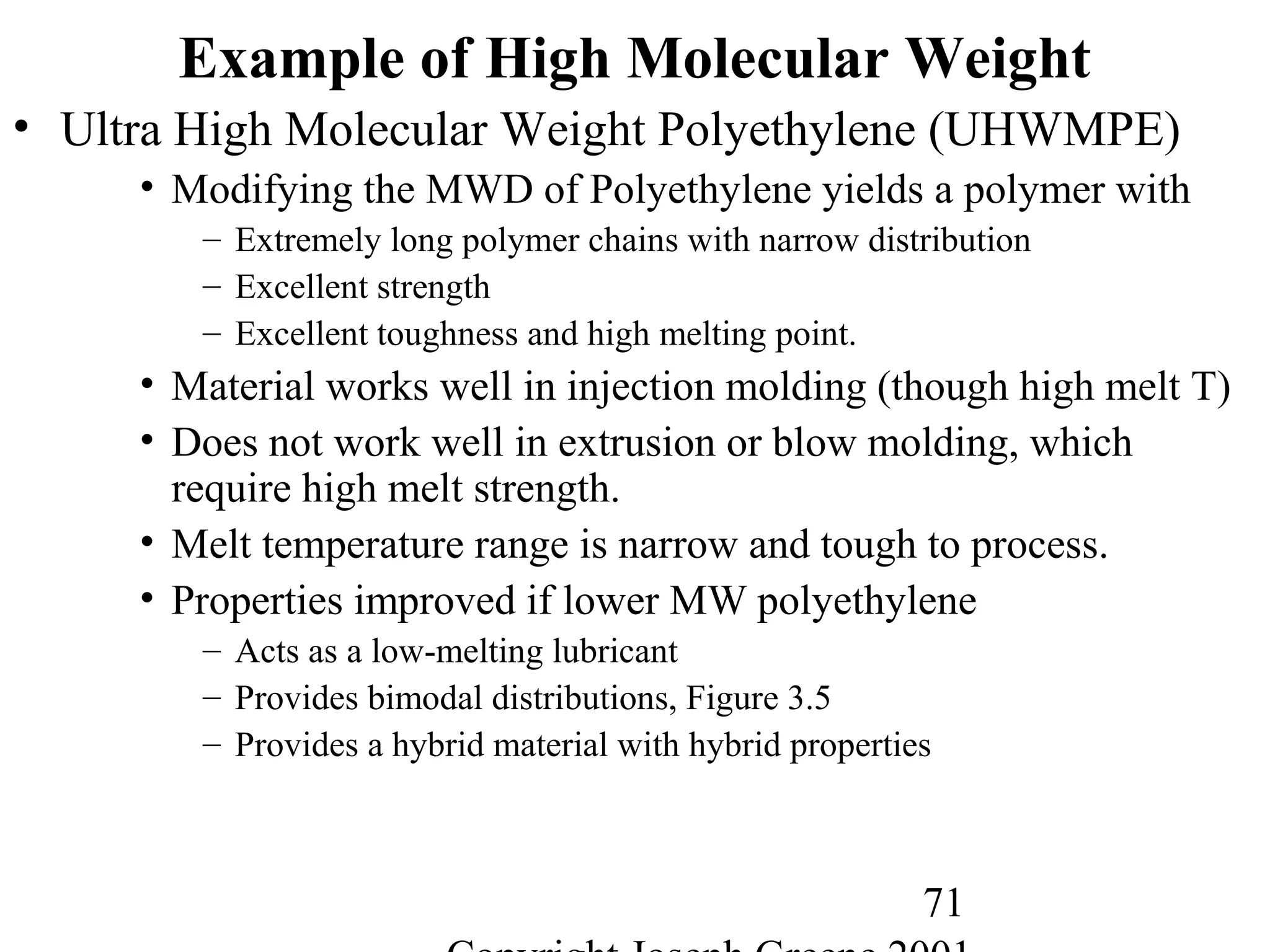
![14
Thermoplastic Elastomers
• Styrene-butadiene rubber (SBR)
– Developed during WWII
• Germany under the name of BUNA-S.
• North America as GR-S,Government rubber-styrene.
– Random copolymer of butadiene (67-85%) and styrene (15-33%)
– Tg of typical 75/25 blend is –60°C
– Not capable of crystallizing under strain and thus requires
reinforcing filler, carbon black, to get good properties.
– One of the least expensive rubbers and generally processes easily.
– Inferior to natural rubber in mechanical properties
– Superior to natural rubber in wear, heat aging, ozone resistance,
and resistance to oils.
– Applications include tires, footwear, wire, cable insulation,
industrial rubber products, adhesives, paints (latex or emulsion)
• More than half of the world’s synthetic rubber is SBR
• World usage of SBR equals natural rubber
C C
H
H H
n
H H
C
H
C
H HH
C C ][](https://image.slidesharecdn.com/m246intro-elast-190727082105/75/ABOUT-ELASTOMER-TYPES-AND-VULCANISATION-14-2048.jpg)
![15
Acrylonitrile-butadiene rubber (NBR)
• Also called Nitrile rubber
– Developed as an oil resistant rubber due to
• the polar C:::N polar bond. Resistant to oils, fuels, and solvents.
– Copolymer of acrylonitrile (20-50%) and butadiene(80-50%)
– Moderate cost and a general purpose rubber.
– Excellent properties for heat aging and abrasion resistance
– Poor properties for ozone and weathering resistance.
– Has high dielectric losses and limited low temperature flexibility
– Applications include fuel and oil tubing; hose, gaskets, and seals; conveyer
belts, print rolls, and pads.
– Carboxylated nitrile rubbers (COX-NBR) has carboxyl side groups
(COOH)which improve
• Abrasion and wear resistance; ozone resistance; and low temperature flexibility
– NBR and PVC for miscible, but distinct polymer blend or polyalloy
• 30% addition of PVC improves ozone and fire resistance
H H
C
H
C
H HH
C C ][C C
H C:::N
H H
n
m](https://image.slidesharecdn.com/m246intro-elast-190727082105/75/ABOUT-ELASTOMER-TYPES-AND-VULCANISATION-15-2048.jpg)
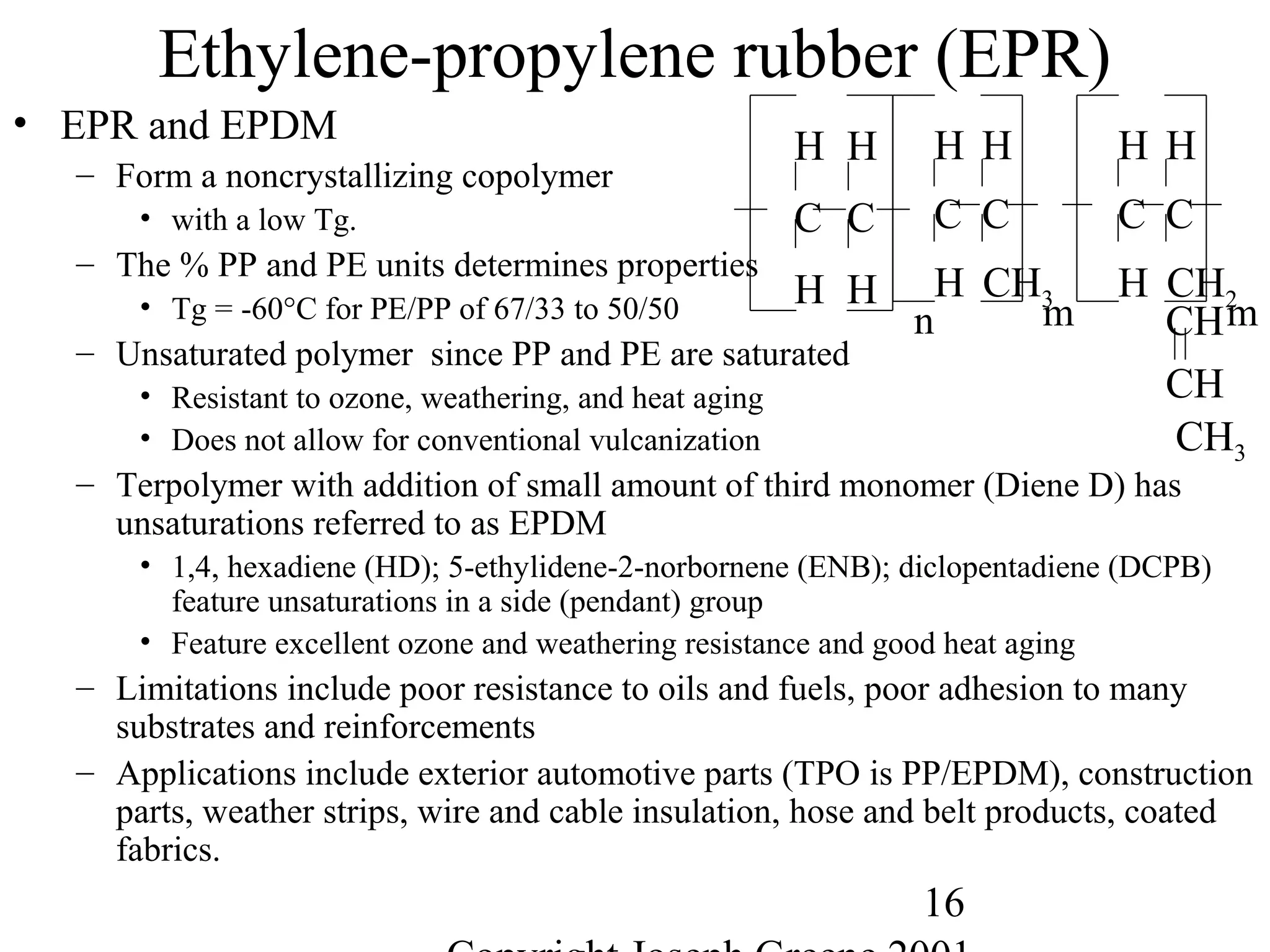
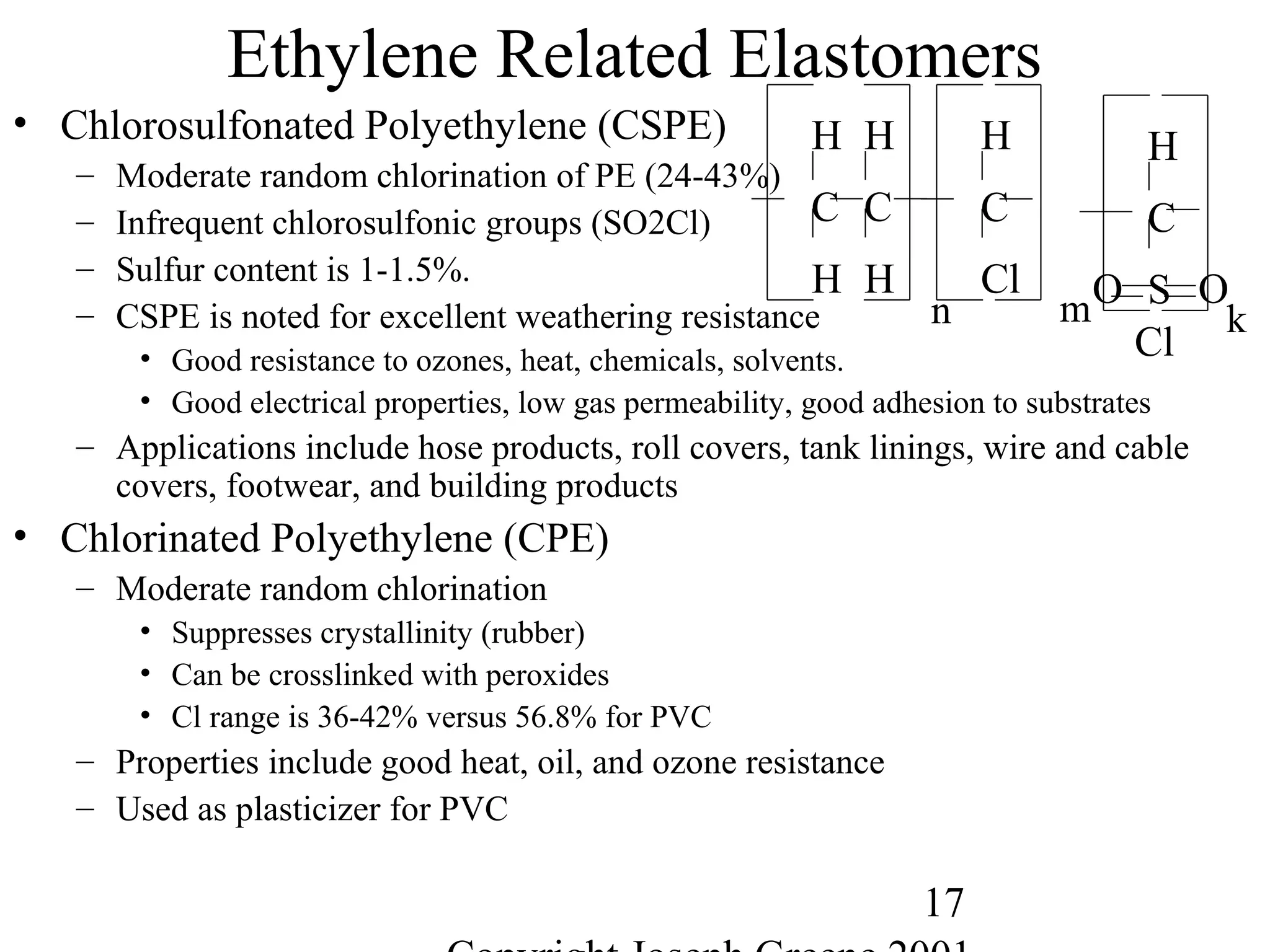
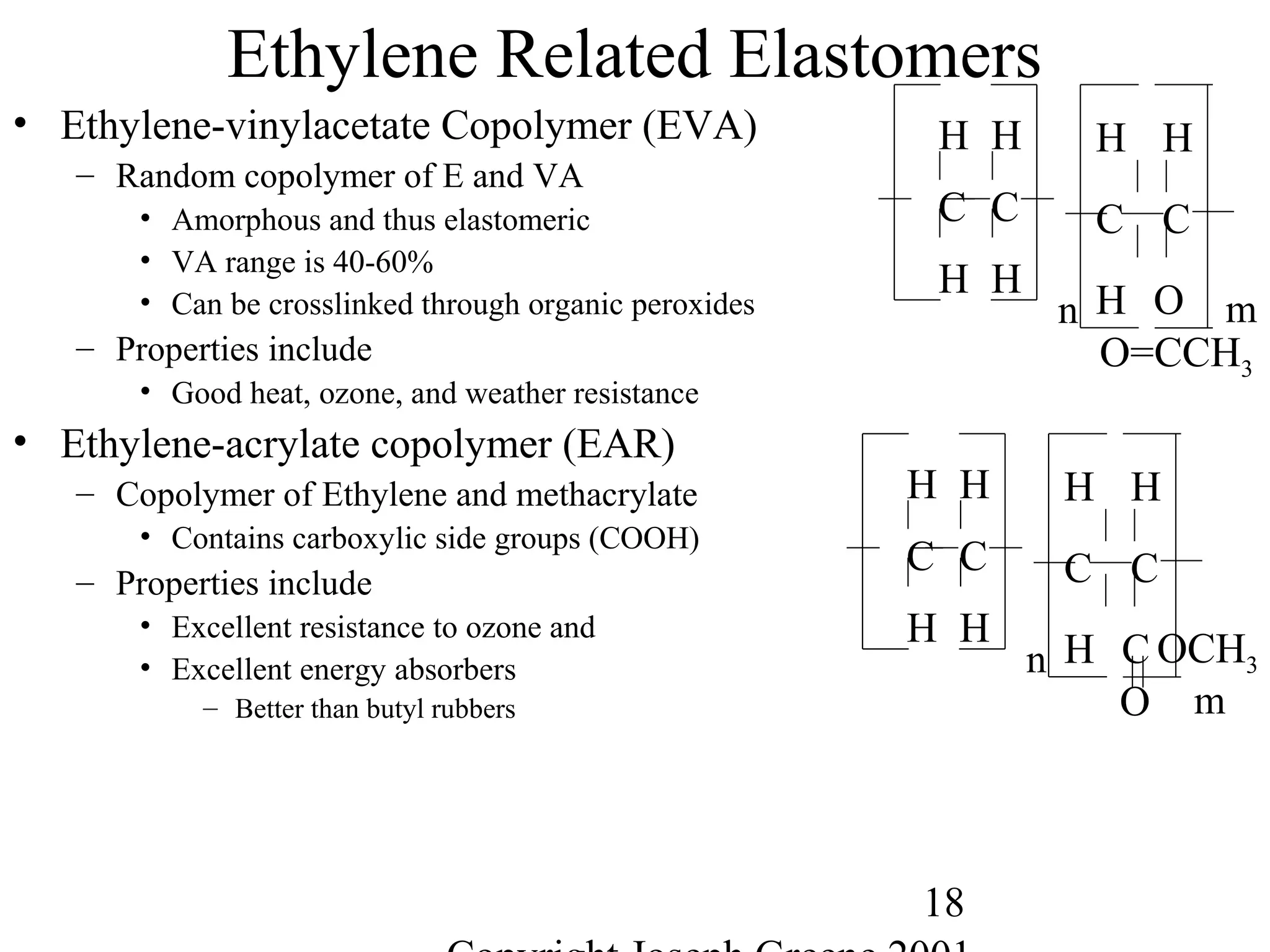
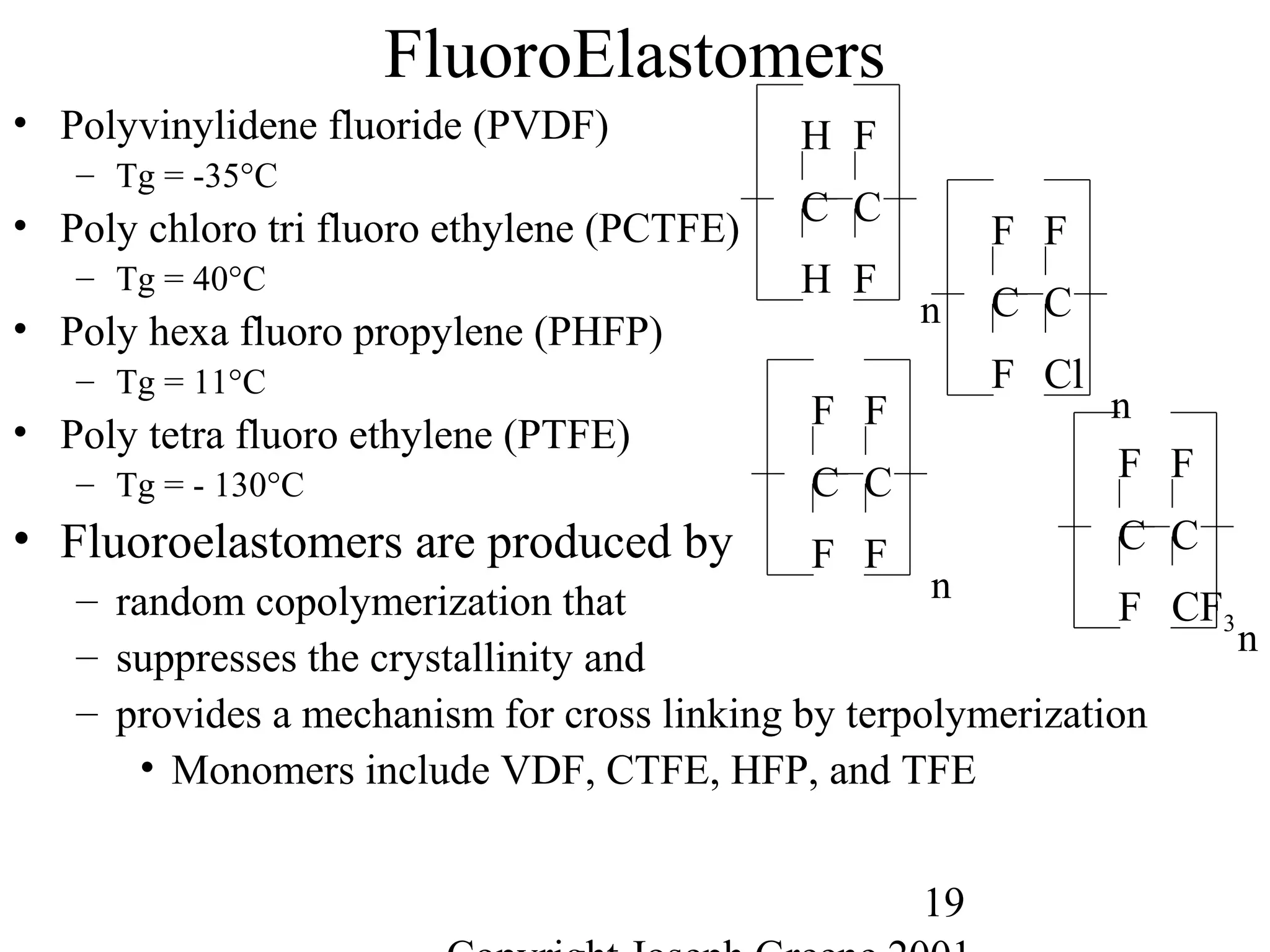


![28
Miscellaneous Other Elastomers
• Polysulfide Rubbers (SR)
– One of the first synthetic rubbers. Tg =-27°C, PES Thiokol A
– Consists of adjacent ethylene and sulfide units giving a stiff chain.
– Flexibility is increased with addition of ethylene oxide for polyethylene-ether-
sulfide (PEES), Thiokol B
– Mechanical properties are not very good, but are used for outstanding resistance
to many oils, solvents and weathering.
– Applications include caulking, mastics, and putty.
• Propylene rubber (PROR)
– Does not crystallize in its atactic form and has a low Tg = -72°C.
– Has excellent dynamic properties
H
C
H
C
H
S S ][
H
S S
C C
H CH3
H H
n
O](https://image.slidesharecdn.com/m246intro-elast-190727082105/75/ABOUT-ELASTOMER-TYPES-AND-VULCANISATION-28-2048.jpg)

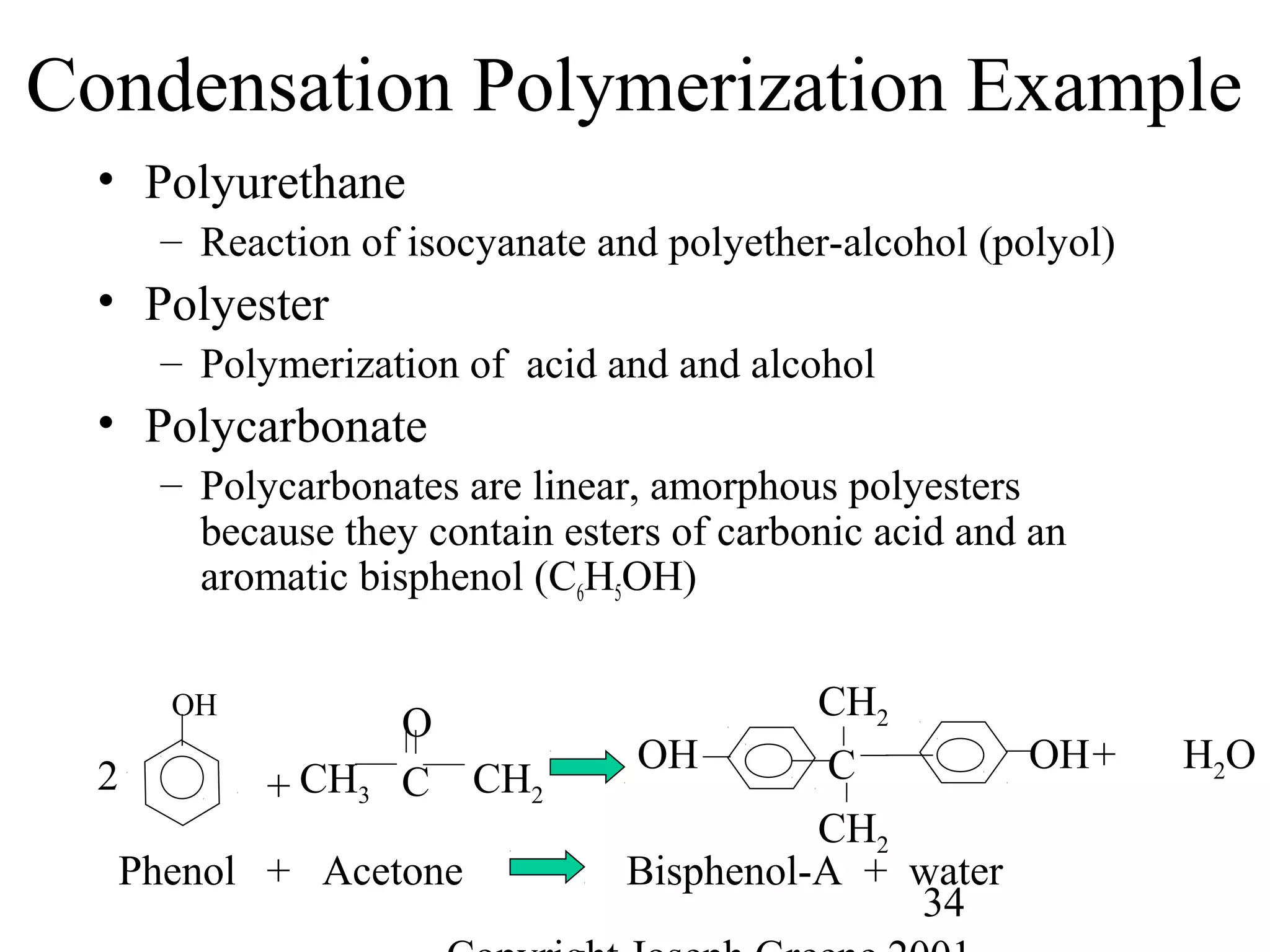
![33
Condensation Polymerization Example
• Polyamides
– Condensation Polymerization
• Nylon 6/6 because both the acid and amine contain
6 carbon atoms
NH2(CH2)6NH2 + COOH(CH2)4COOH
Hexamethylene diamene Adipic acid
n[NH2(CH2)6NH2 ·CO(CH2)4COOH] (heat)
Nylon salt
[NH(CH2)4NH·CO(CH2)4CO]n + nH2O
Nylon 6,6 polymer chain](https://image.slidesharecdn.com/m246intro-elast-190727082105/75/ABOUT-ELASTOMER-TYPES-AND-VULCANISATION-33-2048.jpg)
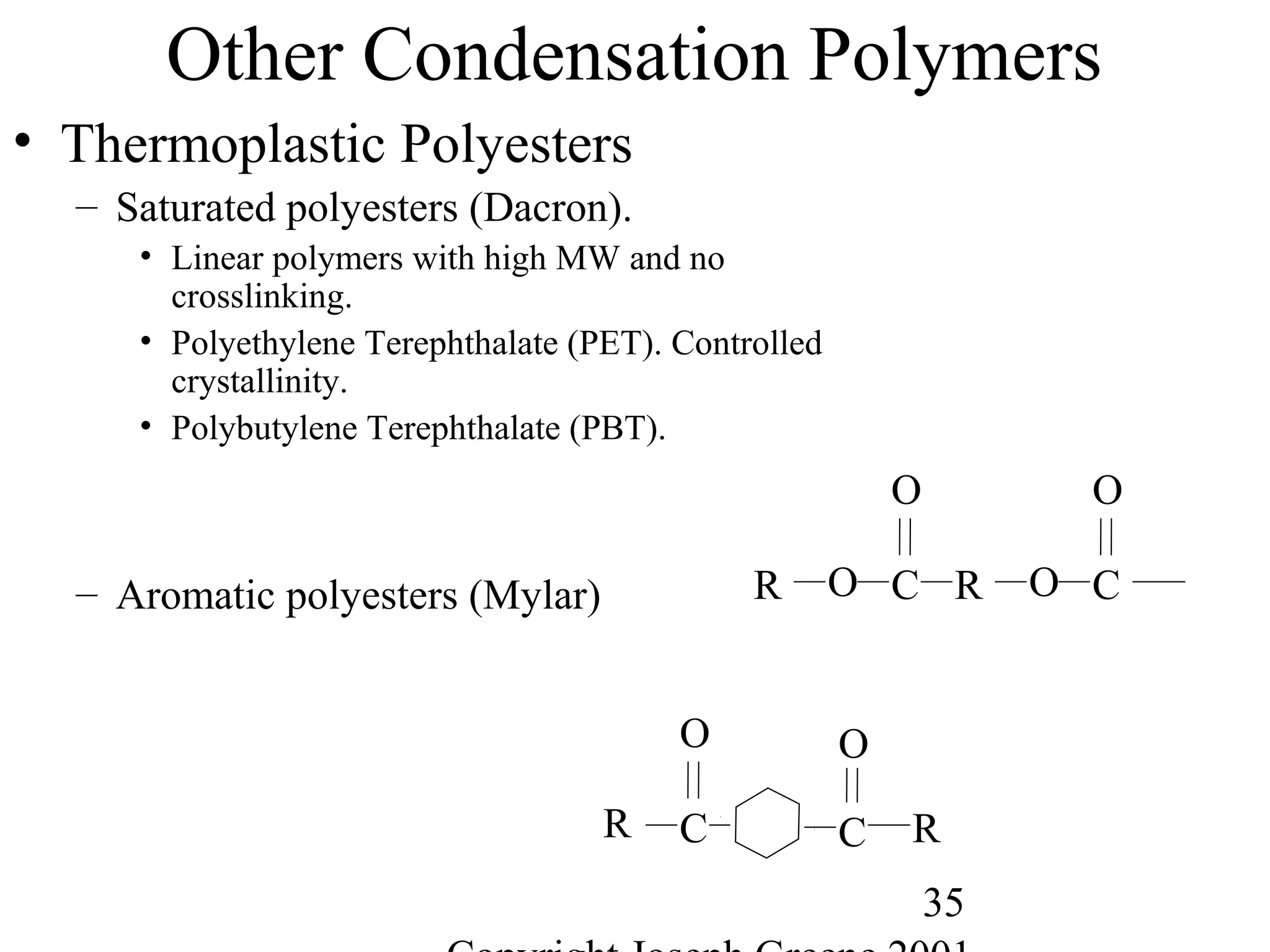
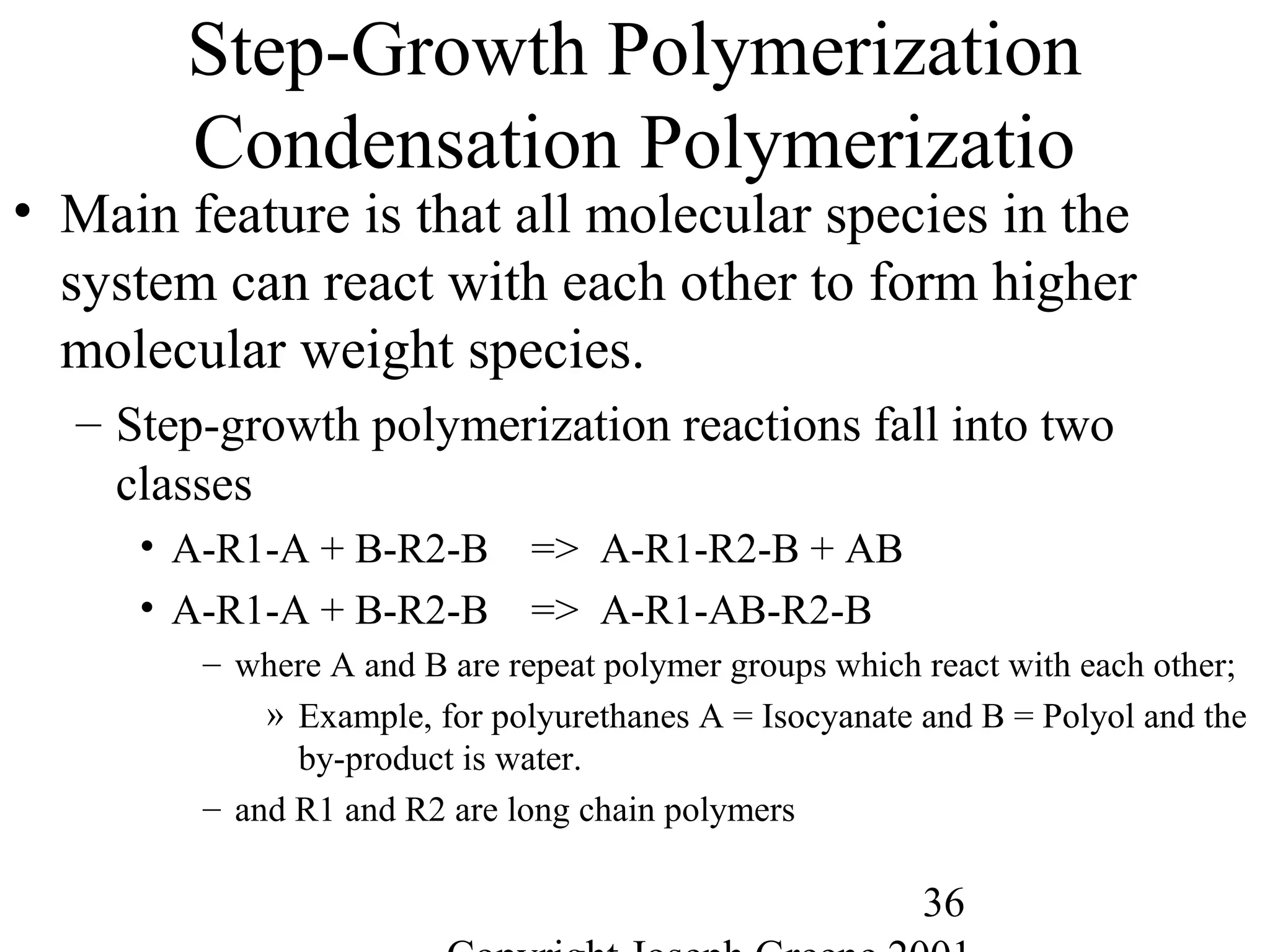




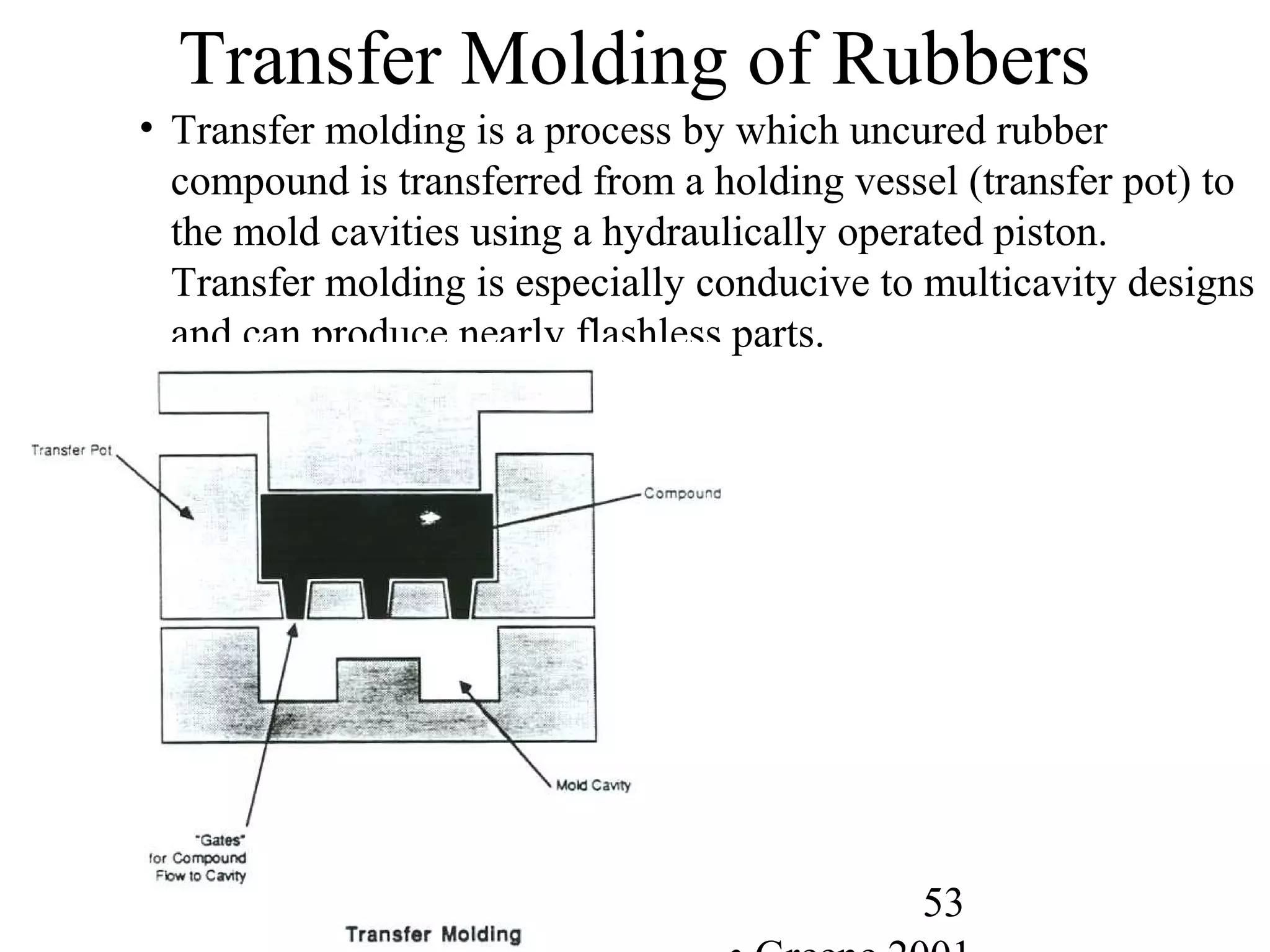
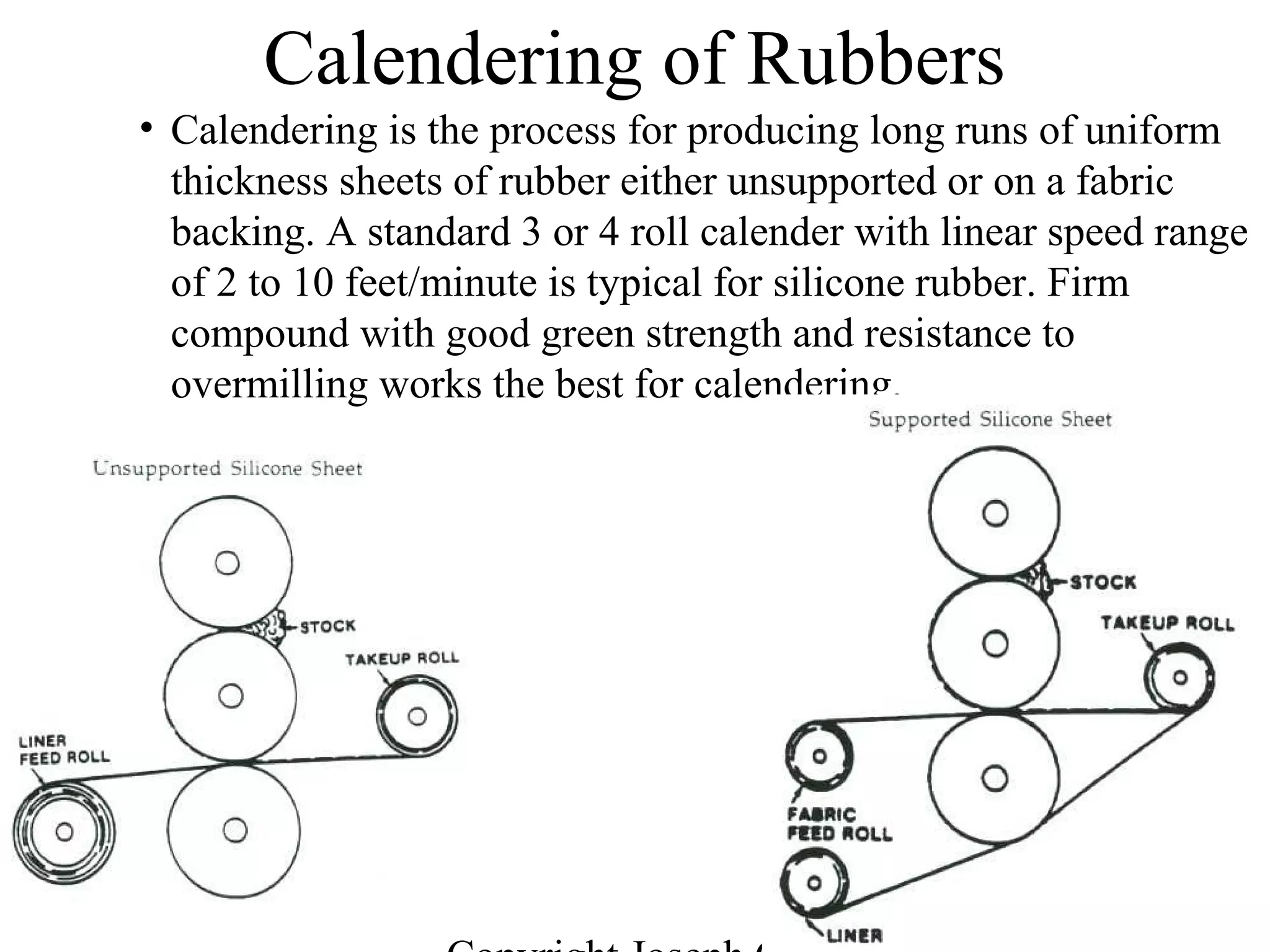

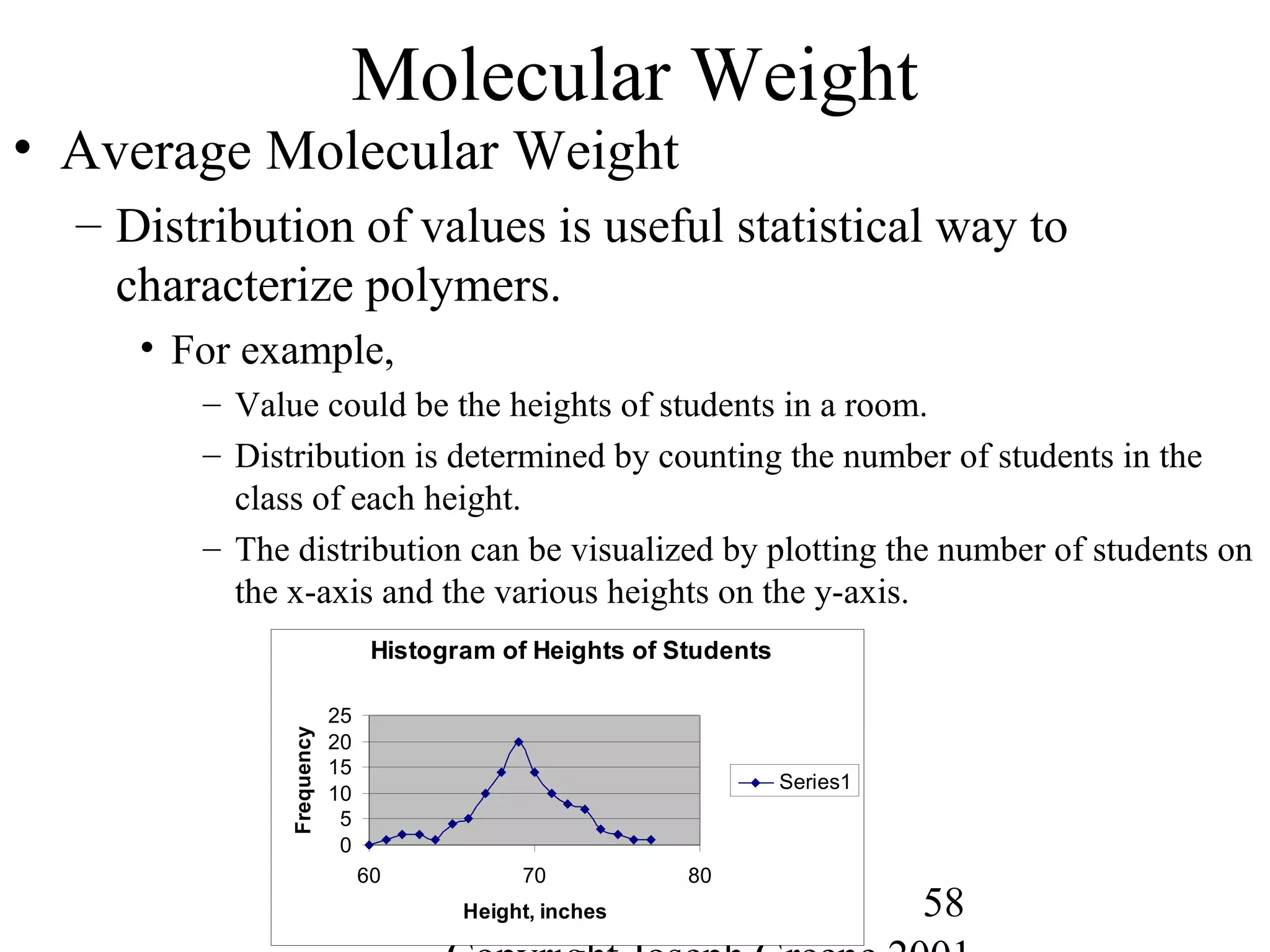
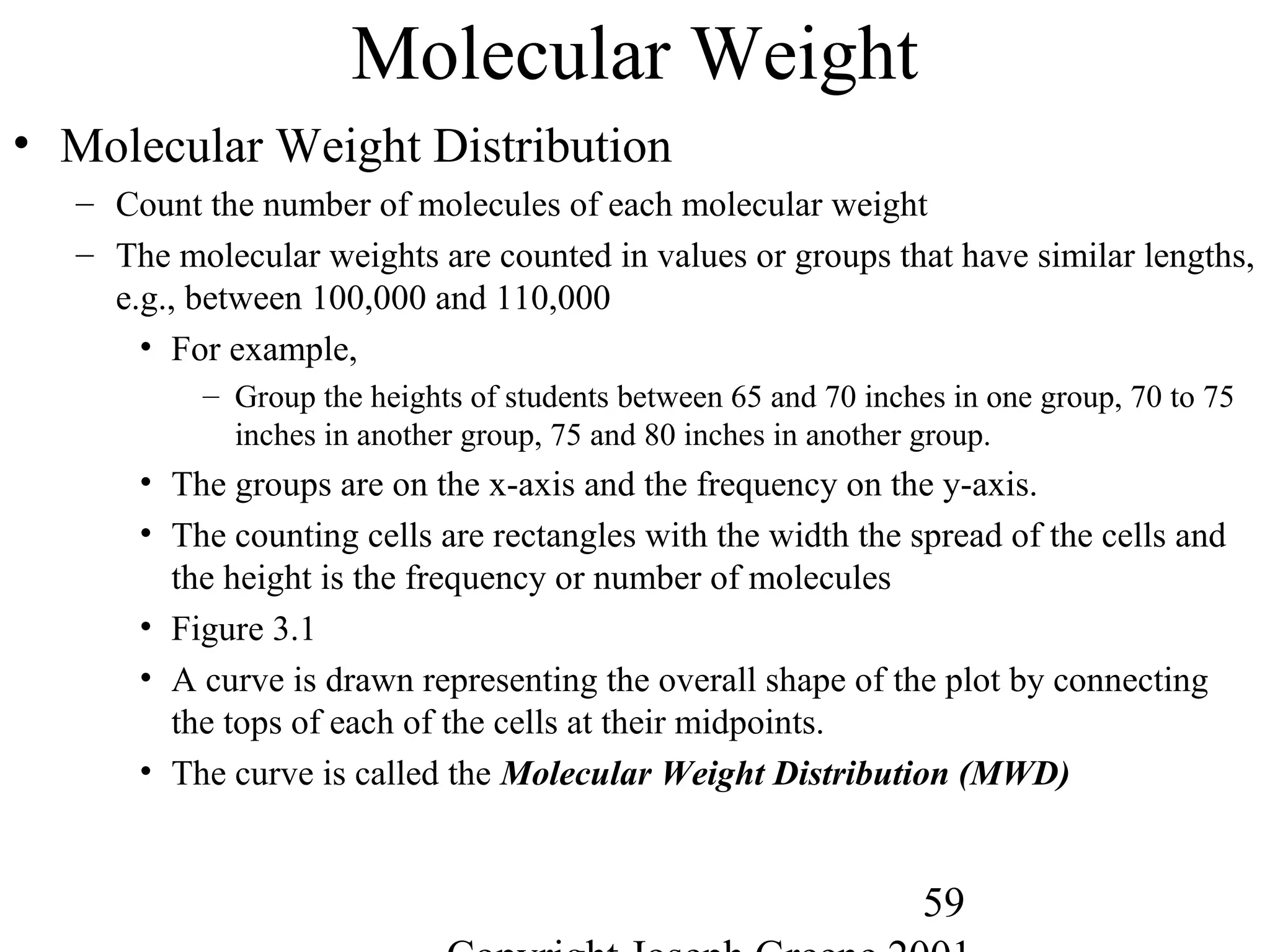
![56
Polymer Length
• Polymer Length
– Polymer notation represents the repeating group
• Example, -[A]-n where A is the repeating monomer and n represents the
number of repeating units.
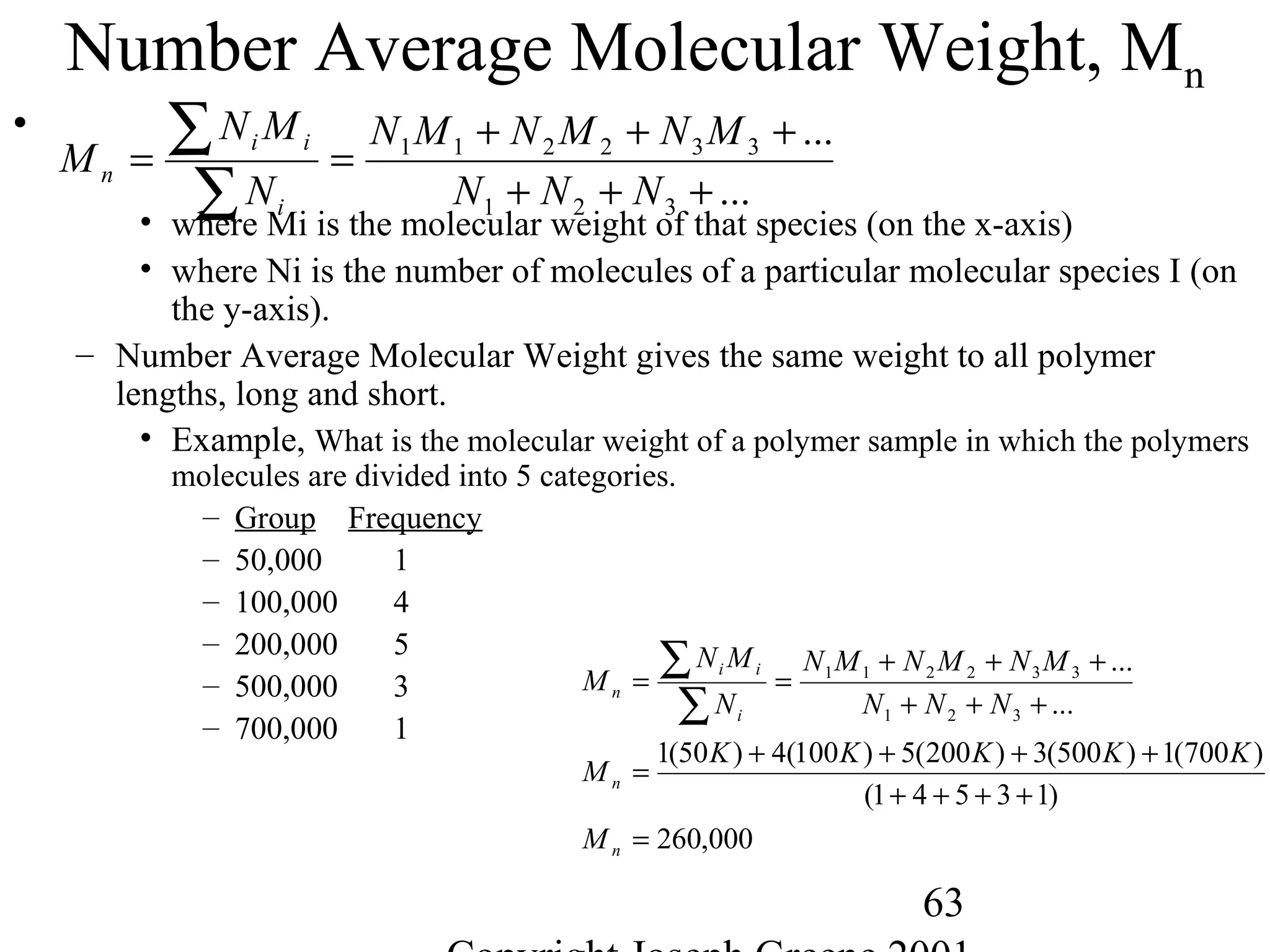
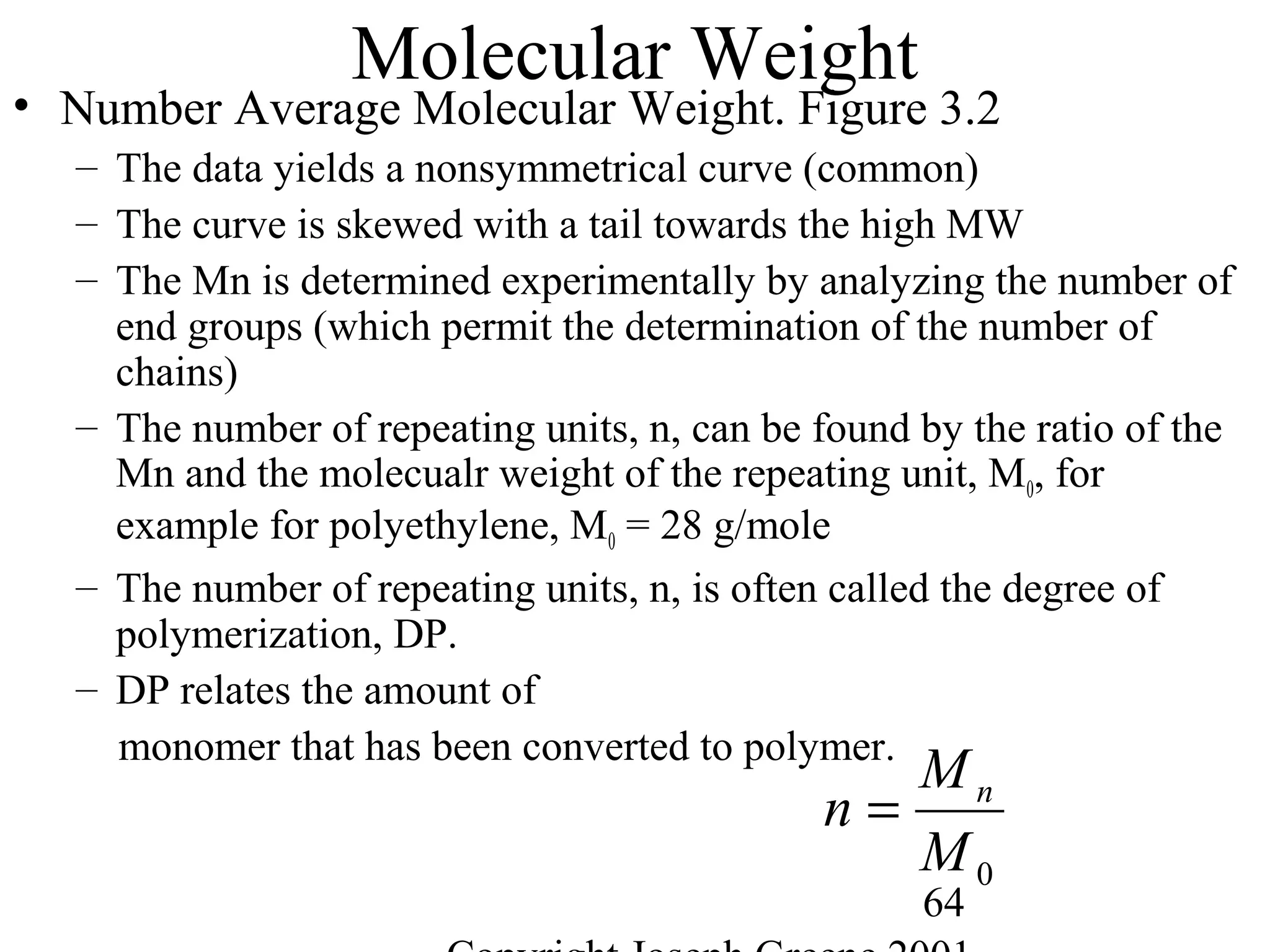
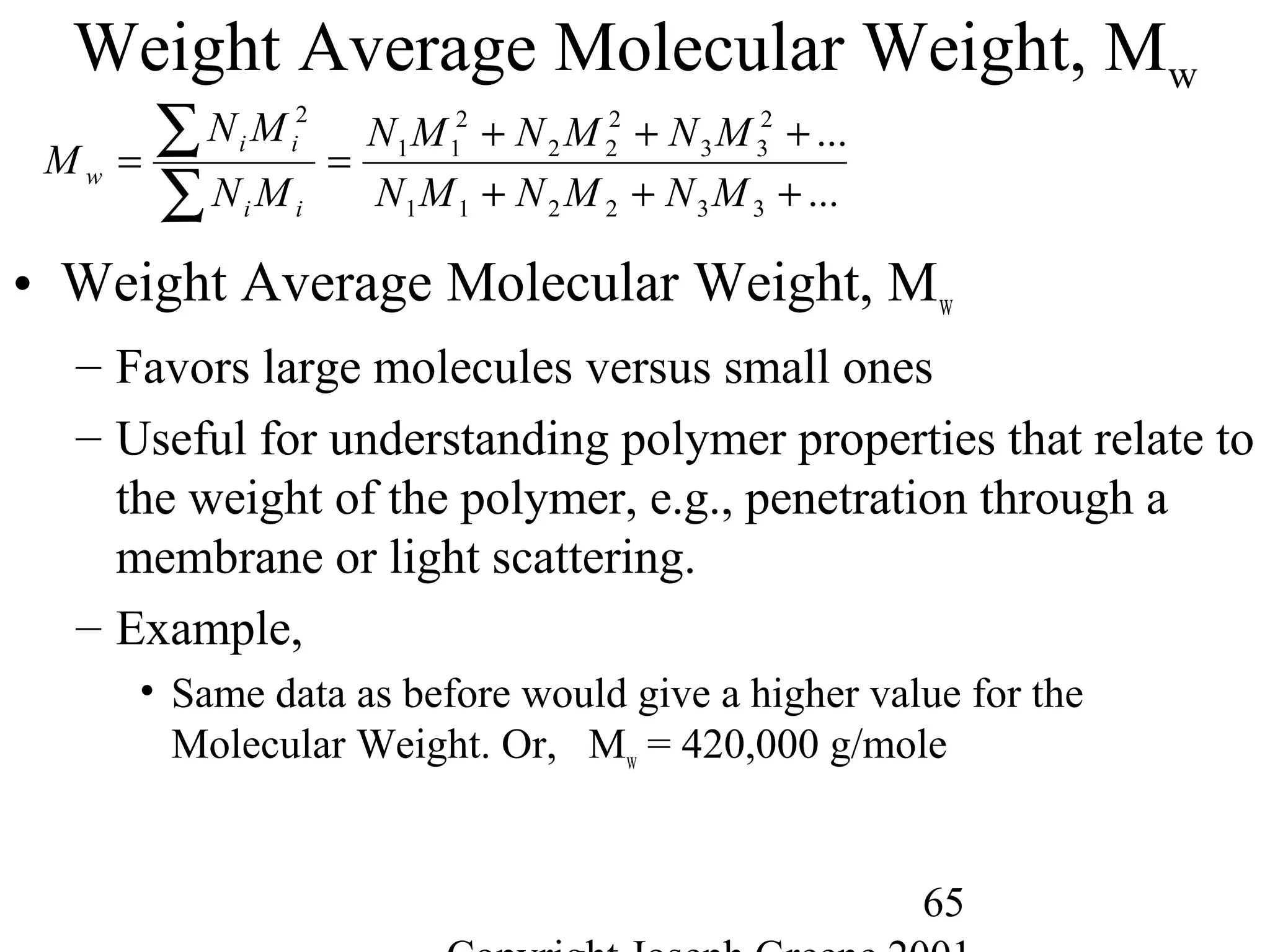
• Molecular Weight
– Way to measure the average chain length of the polymer
– Defined as sum of the atomic weights of each of the atoms in the
molecule.
• Example,
– Water (H2O) is 2 H (1g) and one O (16g) = 2*(1) + 1*(16)= 18g/mole
– Methane CH4 is 1 C (12g) and 4 H (1g)= 1*(12) + 4 *(1) = 16g/mole
– Polyethylene -(C2H4)-1000= 2 C (12g) + 4H (1g) = 28g/mole * 1000 = 28,000
g/mole](https://image.slidesharecdn.com/m246intro-elast-190727082105/75/ABOUT-ELASTOMER-TYPES-AND-VULCANISATION-56-2048.jpg)