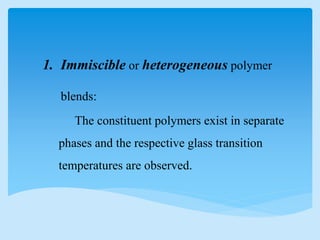
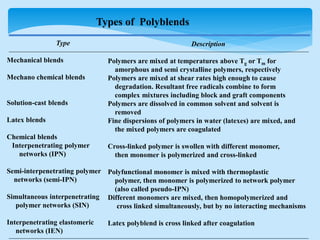
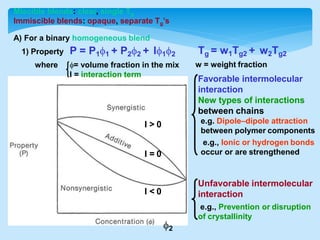
This document discusses polymer blends and alloys. It defines a polymer blend as a mixture of two or more polymers blended to create a new material with different physical properties. There are five main types of polymer blends that are categorized based on miscibility and methods of preparation such as mechanical, solution casting, and latex blends. Polymer blends can improve material properties such as cost, temperature range, toughness, and processability. They are classified as either miscible or immiscible based on whether a single or multiple glass transition temperatures are observed. Compatibilizers and graft copolymerization can be used to improve adhesion between immiscible polymer phases in a blend.