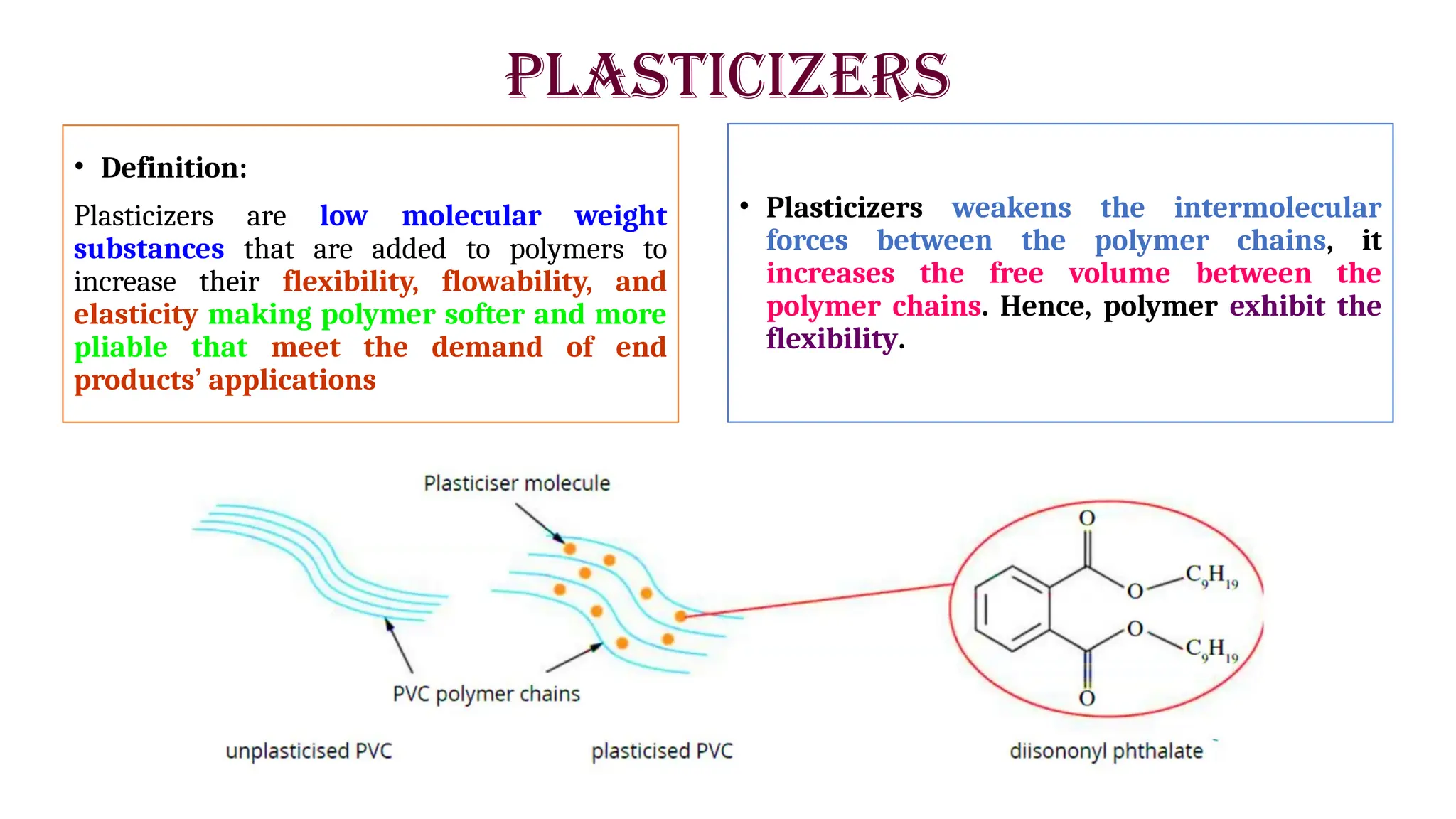
The presentation by Dr. Nandan C. Pomal discusses the evolution and significance of polymer additives in plastic manufacturing, emphasizing their role in improving processability and enhancing the physical properties of polymers. It categorizes different types of additives, including plasticizers, fillers, and stabilizers, and explains their functions and applications in creating flexible, durable, and stable plastic materials. Key points include the importance of non-toxic additives for safety, various examples of plasticizers, and the need for stabilizers to prevent degradation from environmental factors.