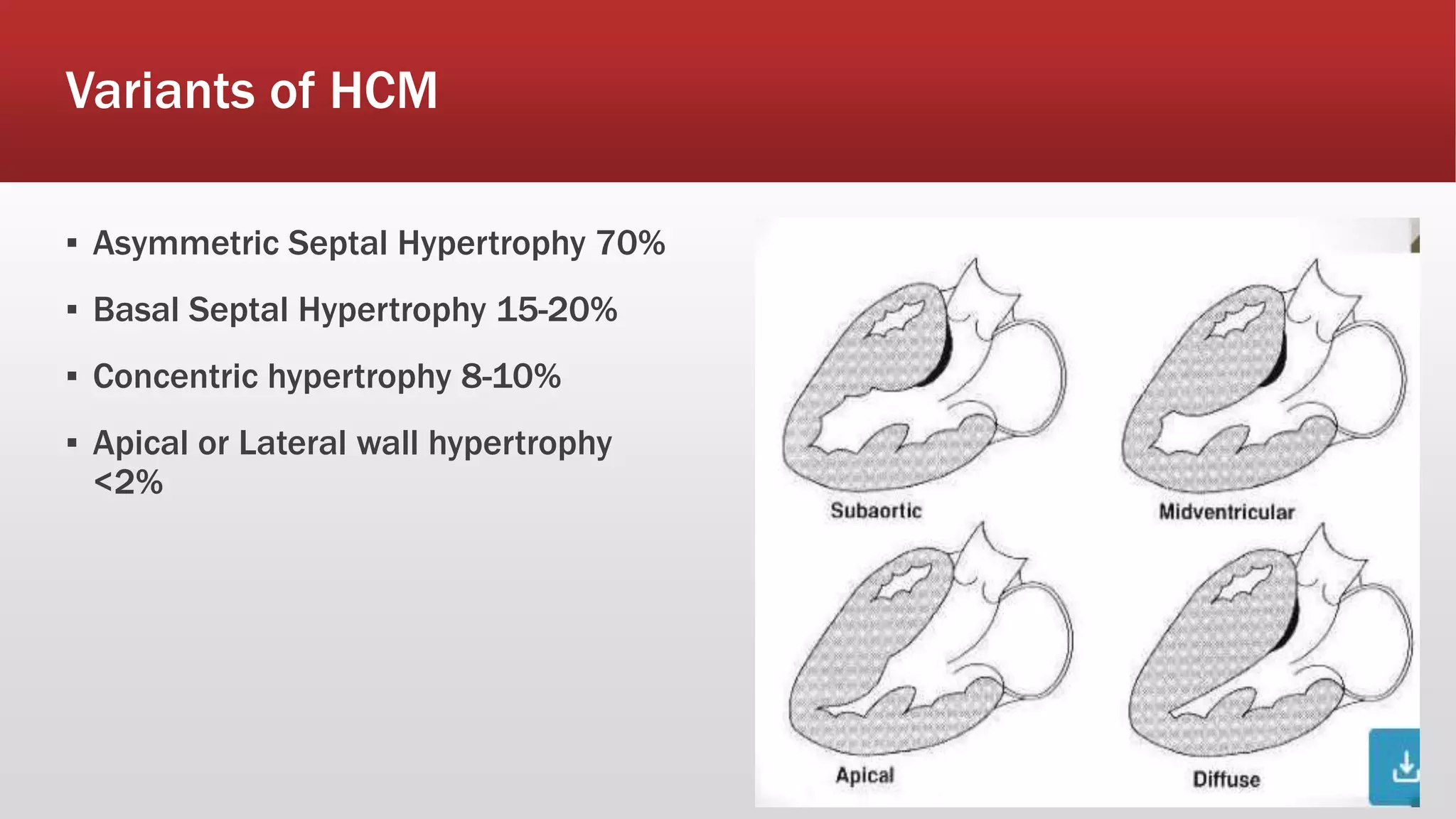
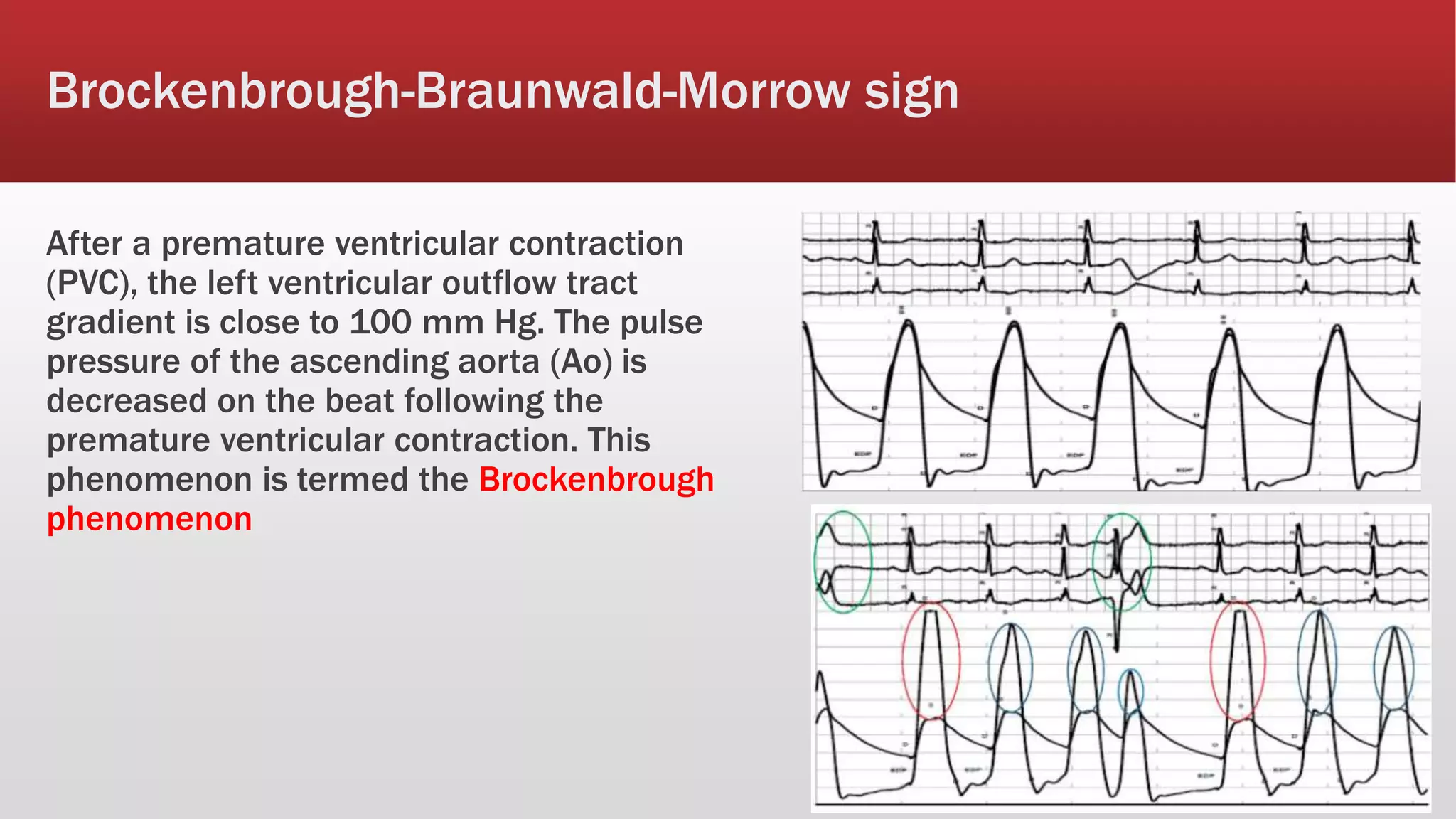
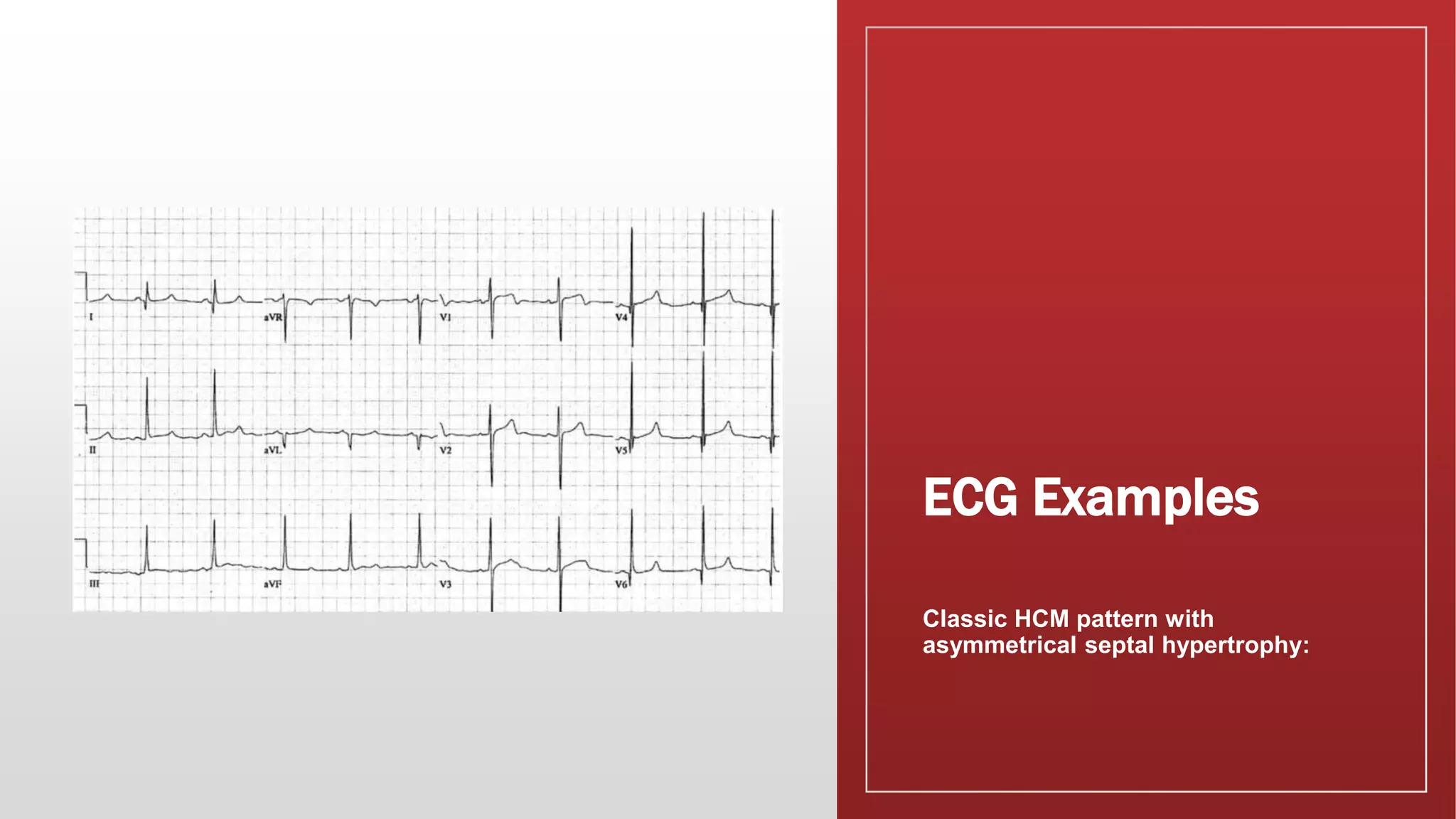
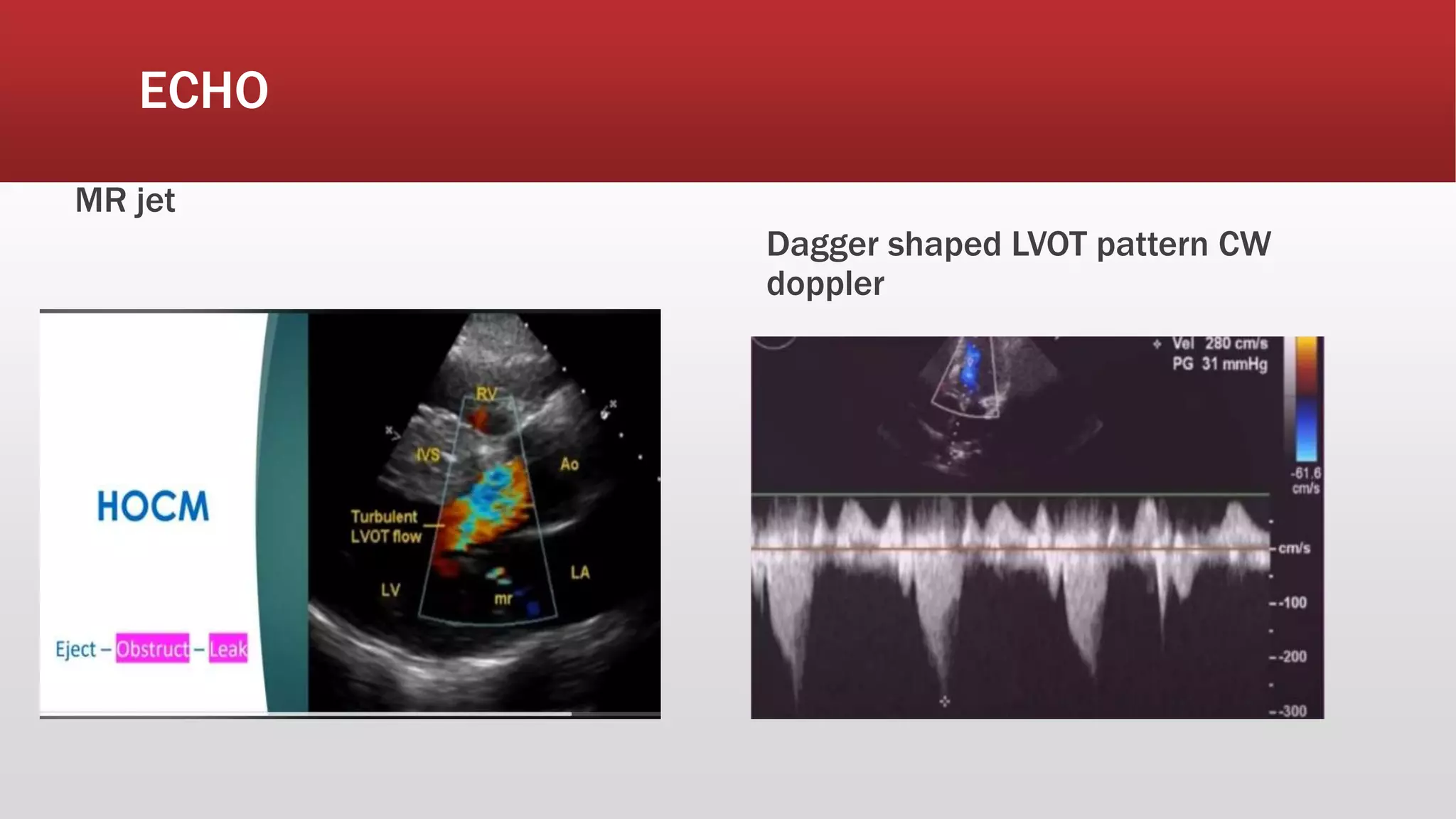
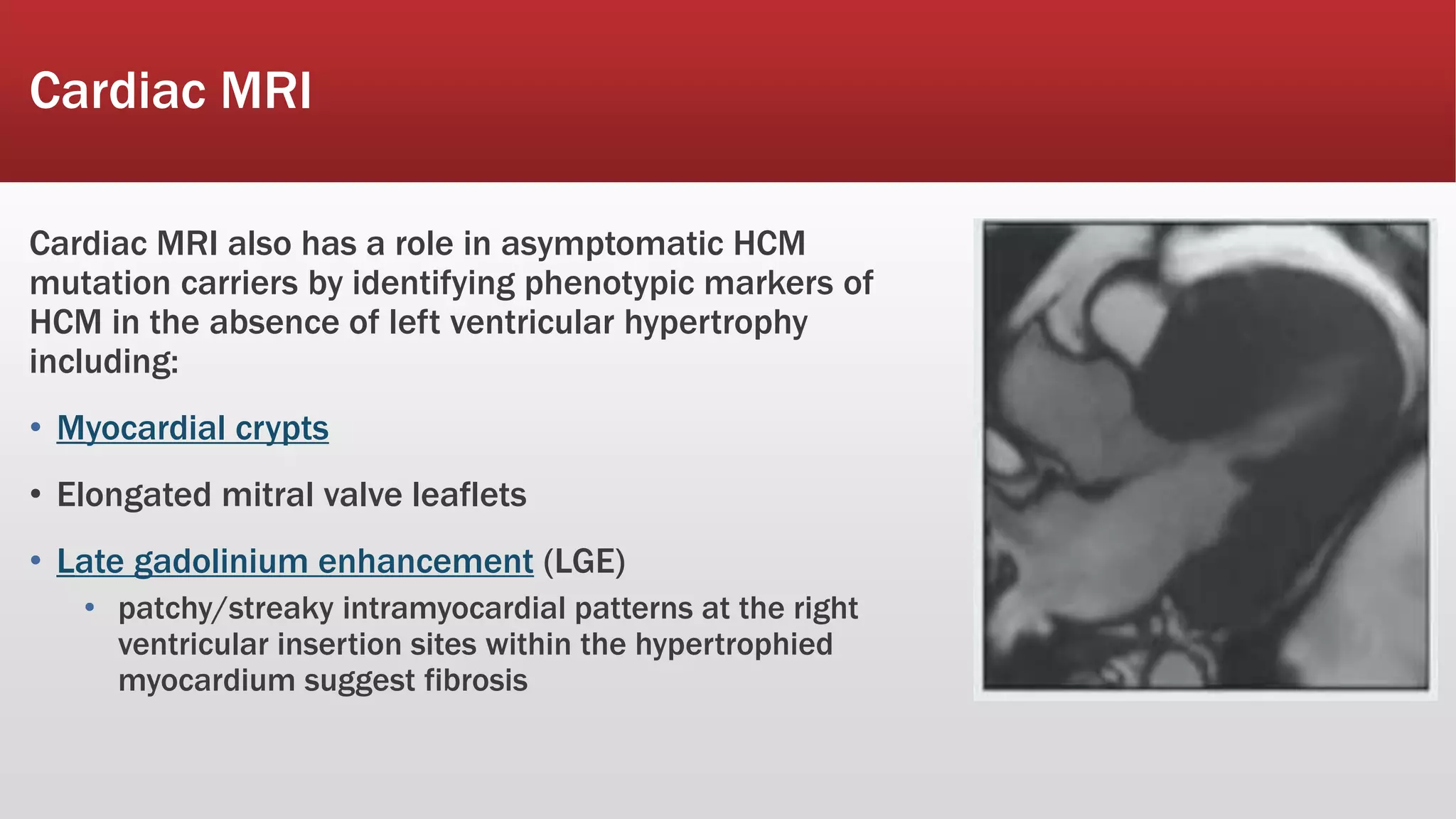
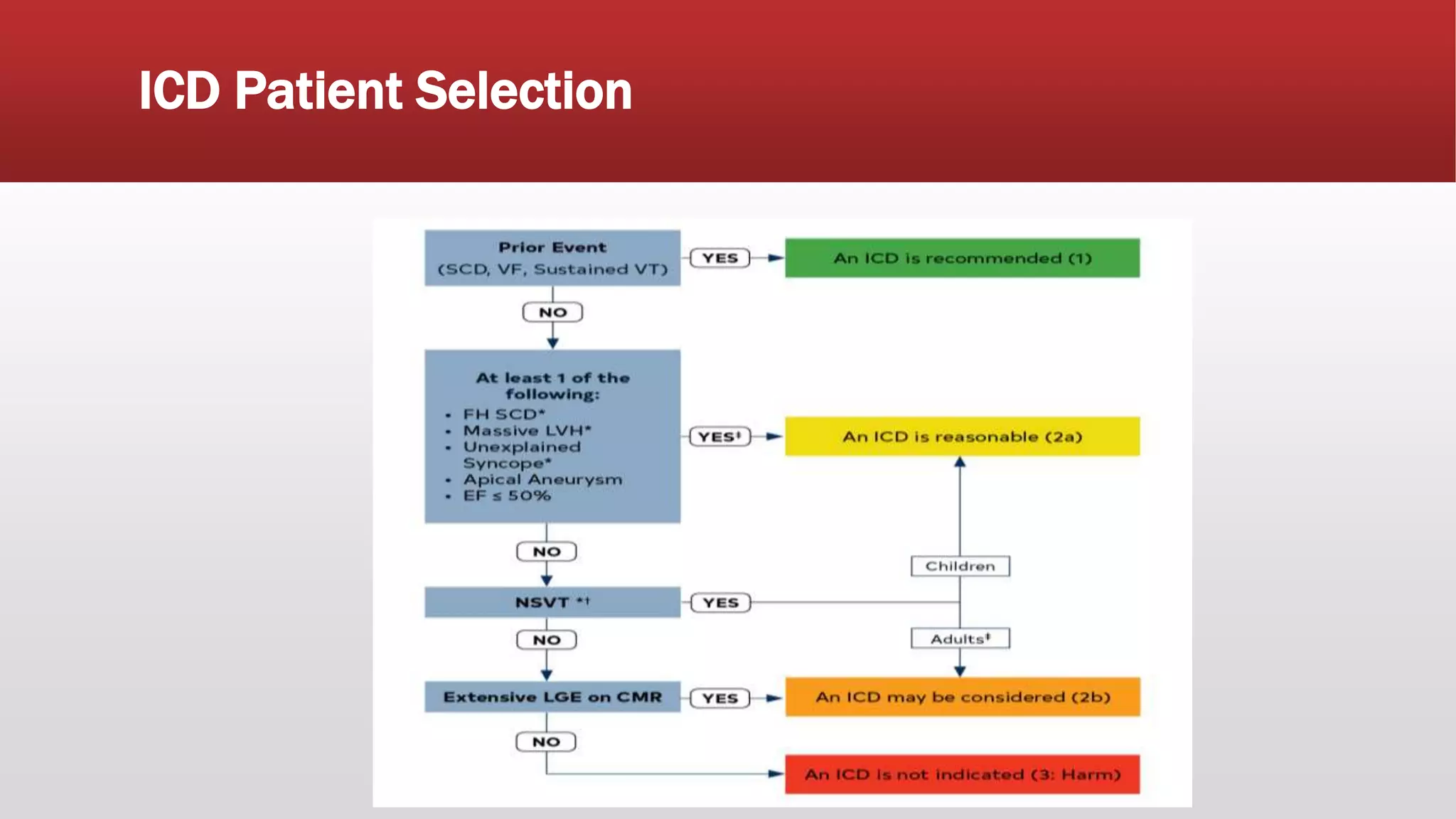
This document summarizes hypertrophic cardiomyopathy (HCM), an autosomal dominant genetic heart condition characterized by unexplained left ventricular hypertrophy. Key points include that it has a prevalence of 1 in 500 adults and is caused by over 200 mutations in genes involved in heart muscle proteins. Symptoms range from none to heart failure, arrhythmias, and sudden cardiac death. Diagnosis is typically made by echocardiogram showing left ventricular hypertrophy. Treatment involves managing symptoms and reducing risk of complications like sudden cardiac death.