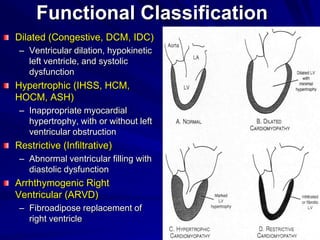
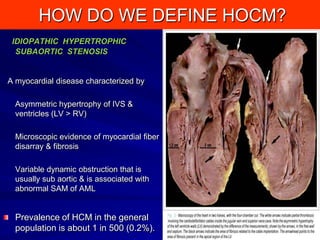
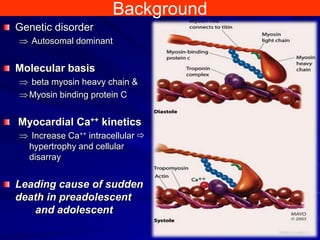
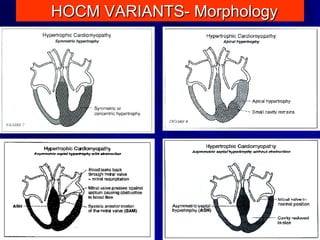
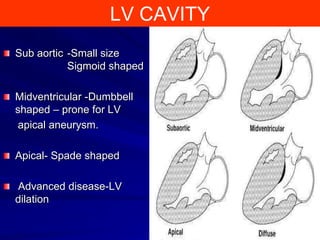
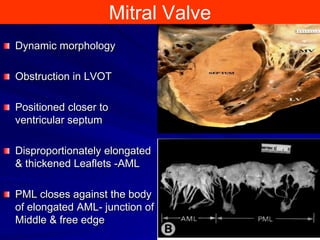
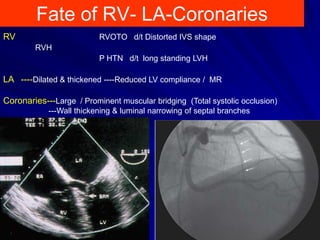
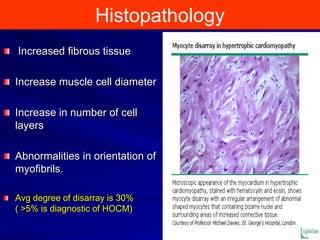
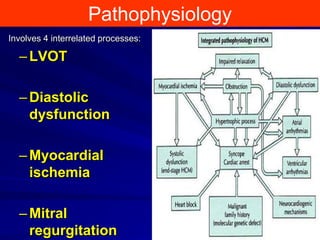
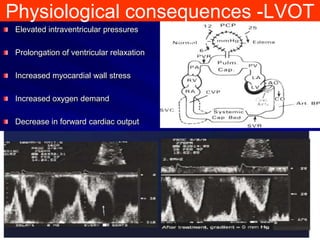
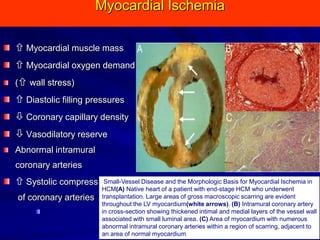
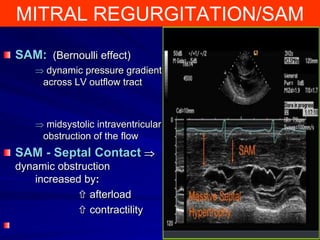
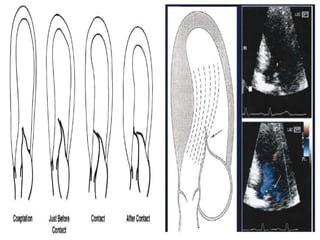
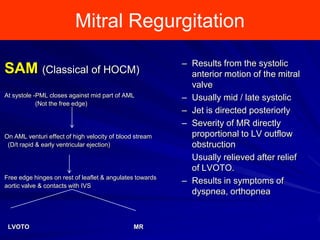
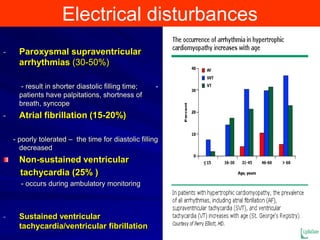
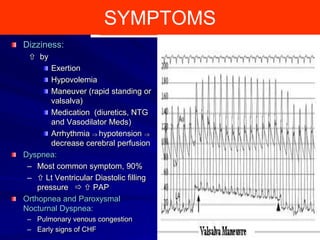
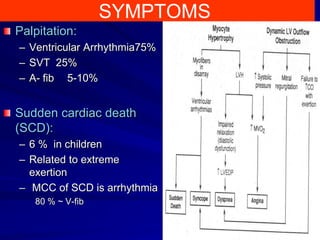
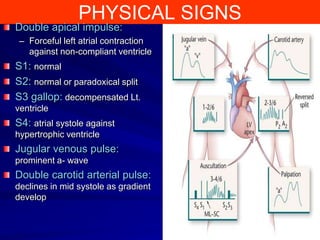
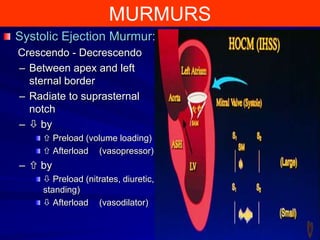
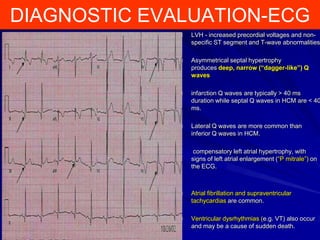
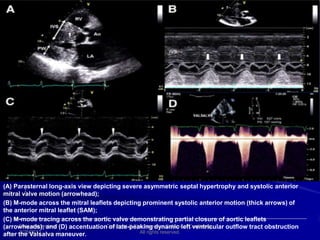
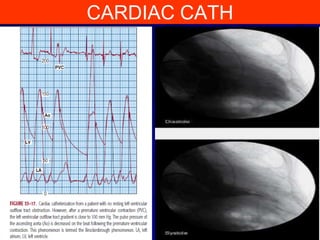
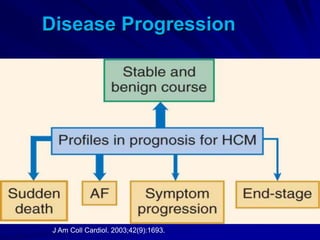
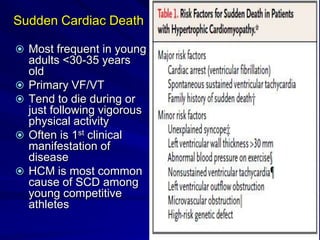
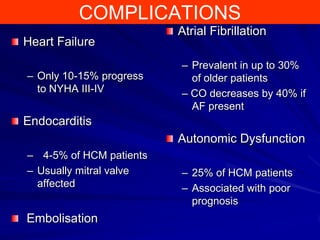
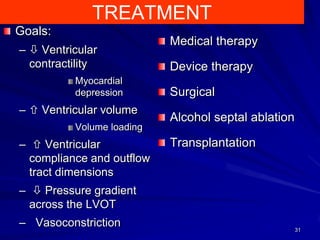
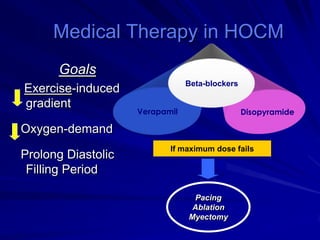
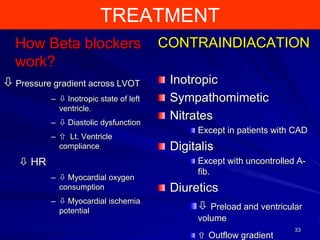
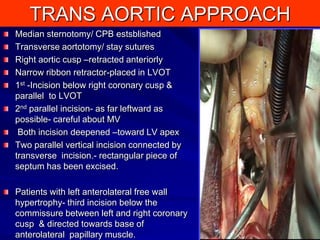
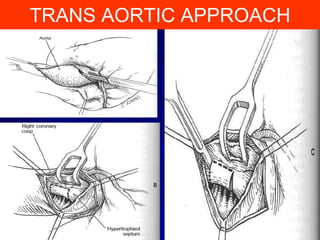
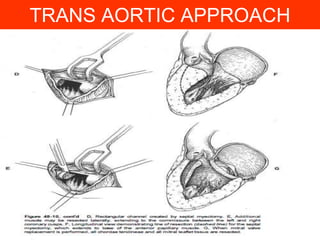
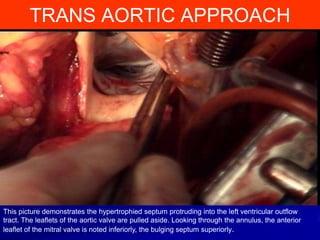
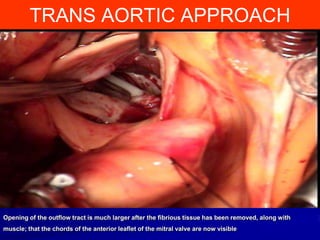
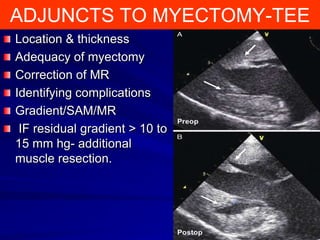
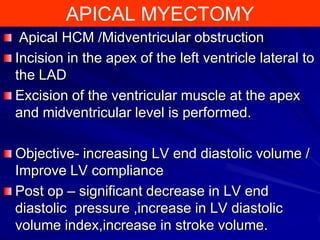
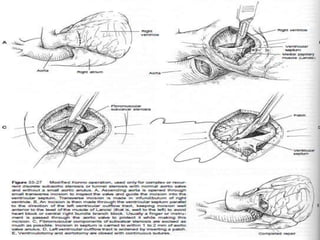
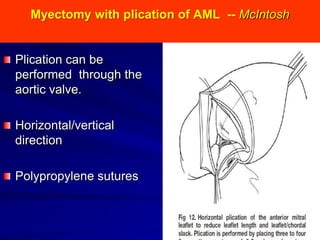
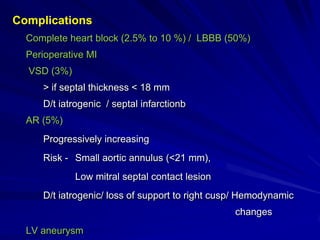
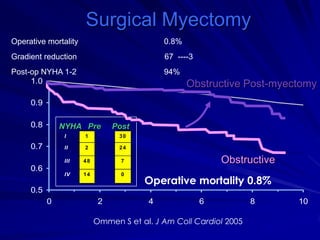
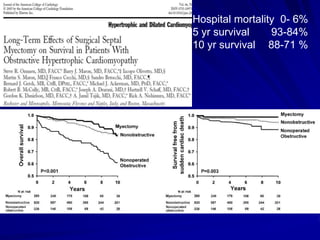
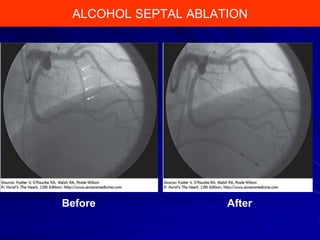
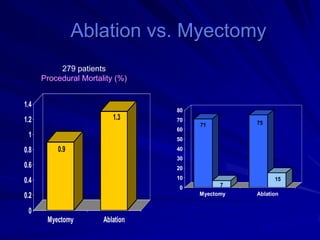
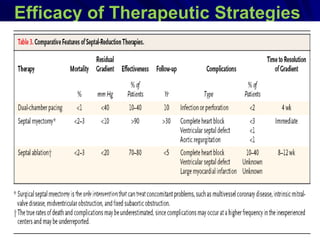
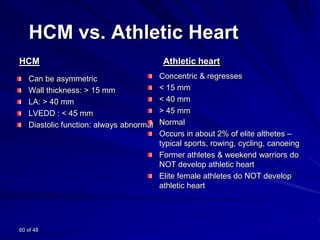
This document contains information about hypertrophic obstructive cardiomyopathy (HOCM). It begins with an overview of HOCM, defining it as a genetic heart condition characterized by asymmetric left ventricular hypertrophy. It then discusses the pathophysiology of HOCM, focusing on left ventricular outflow tract obstruction, diastolic dysfunction, myocardial ischemia, and mitral regurgitation due to systolic anterior motion of the mitral valve. The document outlines clinical manifestations such as symptoms, physical exam findings, ECG and echocardiographic features, and complications. It concludes by covering treatment options for HOCM including medications, surgical septal myectomy via transaortic or transapical approaches, and other procedures like alcohol septal