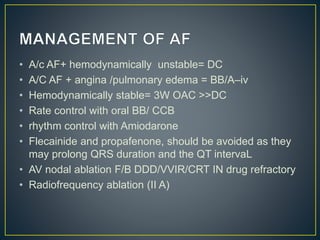
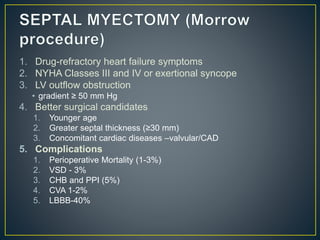
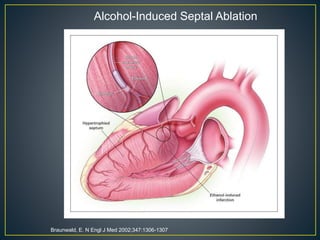
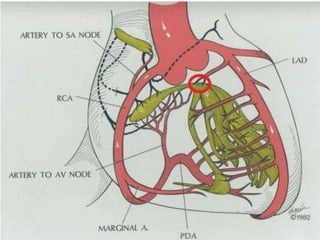
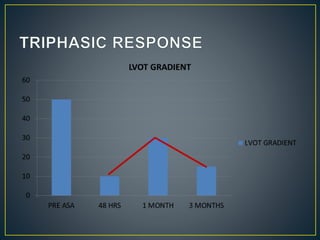
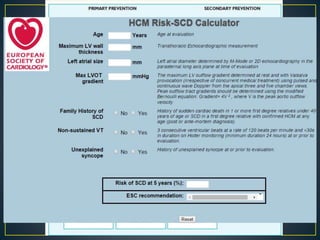
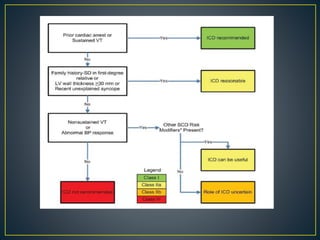
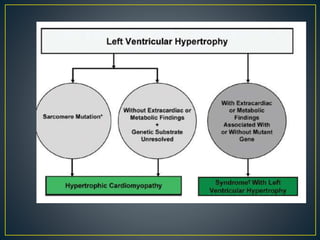
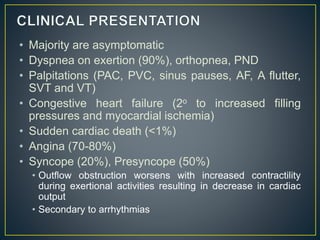
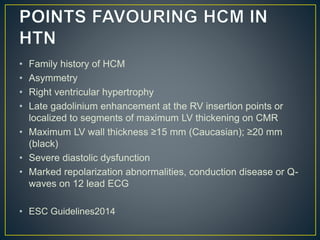
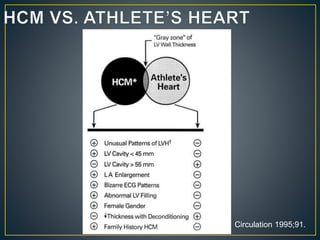
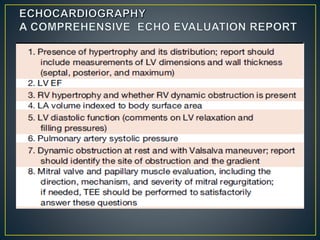
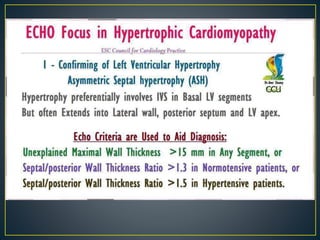
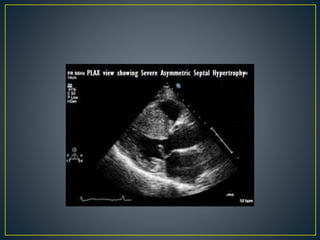
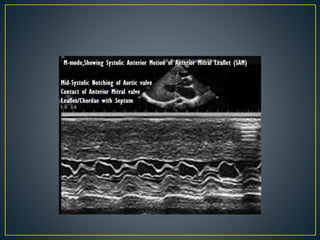
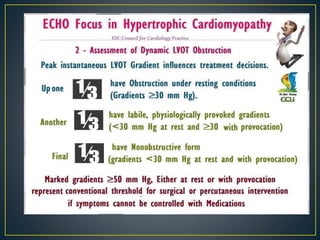
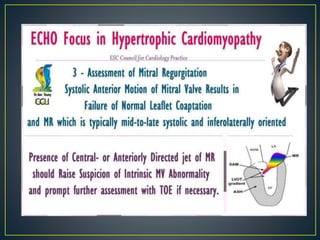
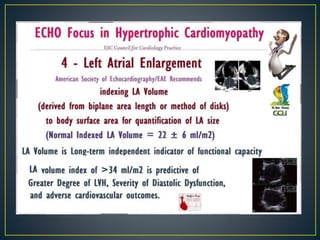
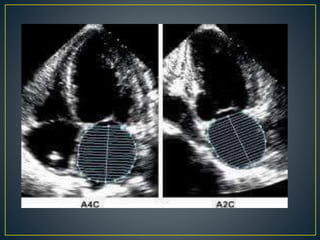
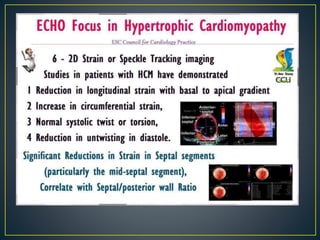
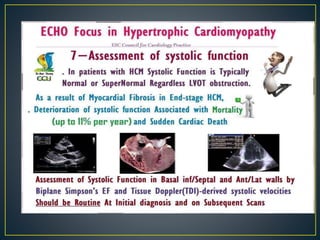
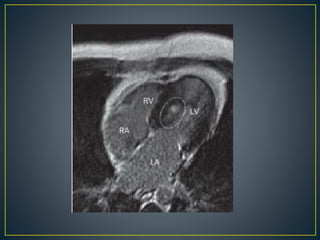
Hypertrophic cardiomyopathy (HCM) is a genetic condition marked by unexplained left ventricular (LV) hypertrophy, commonly presenting with symptoms such as exertional dyspnea and palpitations. Diagnosis utilizes echocardiography, MRI, and genetic testing, with management depending on the severity of obstruction and symptoms, including beta-blockers, calcium channel blockers, and potential surgical interventions. Risk stratification is crucial, particularly for family members, as sudden cardiac death can occur in patients with HCM, emphasizing the need for regular monitoring and management strategies.




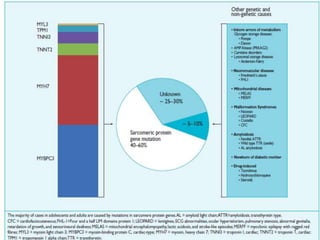















![Heart failure
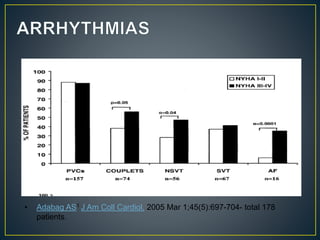
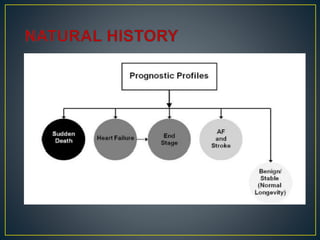
NYHA III - IV [10 - 15 %]
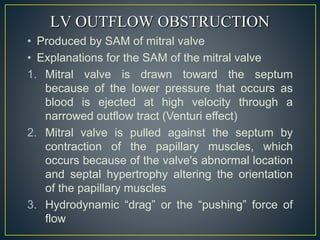
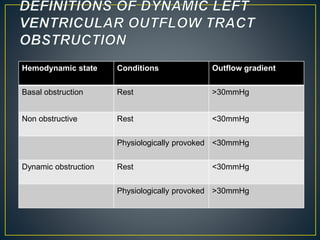
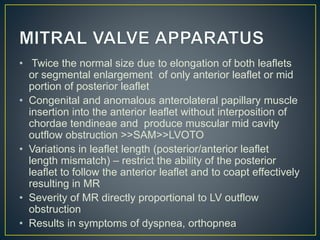
• LV outflow obstruction
• AF
• Diastolic dysfunction
• Micro vascular dysfunction](https://image.slidesharecdn.com/hocmdrpritam-171106181120/85/Hypertrophic-Cardiomyopathy-52-320.jpg)