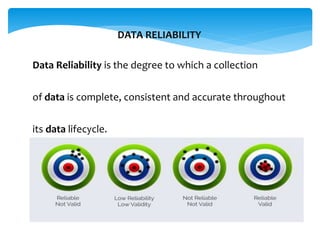
This document provides information on good documentation practices (GDP) for pharmaceutical manufacturing. It discusses how documentation is crucial for quality assurance and aims to ensure manufacturing processes are carried out consistently as planned. It defines key terms like documents, records, data reliability and integrity. The document outlines good practices for documentation like contemporaneous recording, error correction procedures, and preventing issues like falsification. It provides examples of documentation deficiencies from inspections and consequences of poor practices like warning letters. Maintaining ALCOA principles of documentation - being Attributable, Legible, Contemporaneous, Original and Accurate - is emphasized. Case studies on meningitis and salmonella outbreaks show how documentation lapses can have severe public health impacts.





















![ ALCOA was coined by Stan Woollen in the early
1990’s.
1999 FDA Guidance: ALCOA - “To be acceptable the
data [from clinical trials] should meet certain
fundamental elements of quality whether collected
or recorded electronically or on paper. Data should
be Attributable, Legible, Contemporaneous,
Original, and Accurate”
ALCOA](https://image.slidesharecdn.com/gdp-alcoa-160229041605-converted-190824102526/85/Gdp-alcoa-160229041605-converted-23-320.jpg)