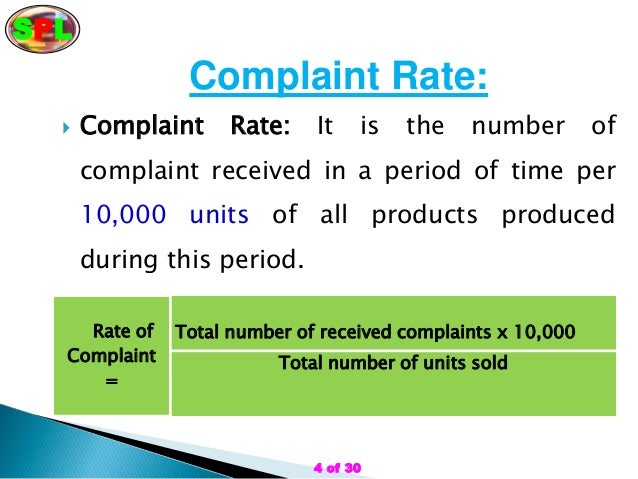
The presentation outlines the definition and importance of customer complaints in the pharmaceutical industry, highlighting doctors as primary customers and explaining that complaints can indicate dissatisfaction with product quality. It describes various types of complaints, including quality, adverse reactions, and medically related issues, as well as the protocols for handling complaints to ensure good manufacturing practices. The complaint handling process involves multiple departments working together to investigate and address issues, ultimately aiming to improve product quality and customer relationships.