Embed presentation
Downloaded 103 times





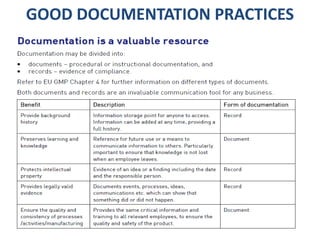


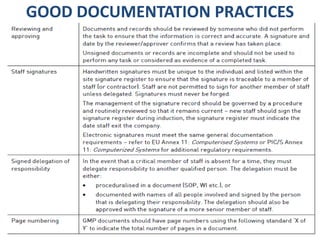

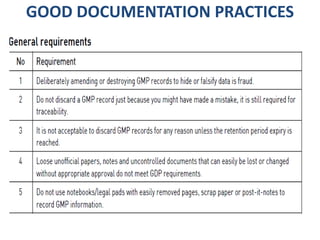



This document discusses good documentation practices for the pharmaceutical industry. It explains that documentation needs to meet certain standards to ensure product quality and safety. Poor documentation can negatively impact manufacturing and quality assurance, potentially reducing patient safety. The regulations from PIC/S, FDA, and EU all include mandatory sections on documentation, as it provides information on tasks and evidence that tasks were completed properly. Following good documentation standards can help companies successfully manufacture quality, safe products and improve audit outcomes.












