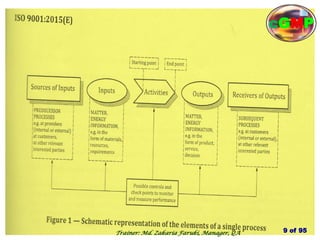
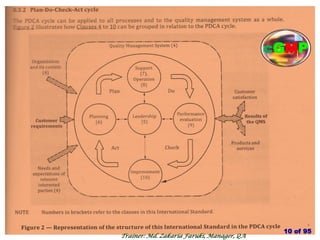
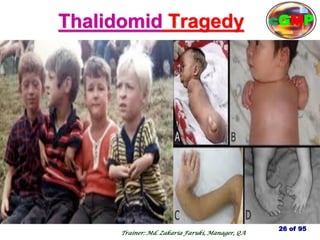
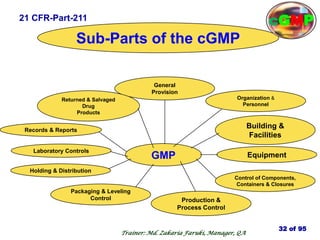
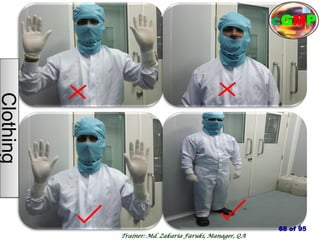
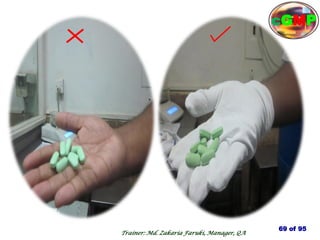
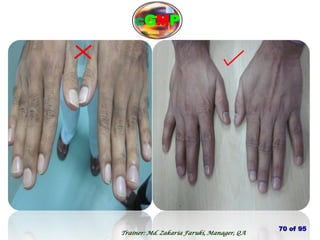
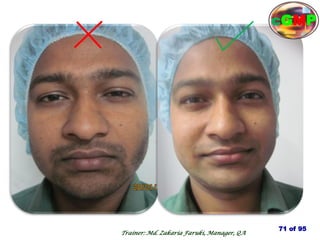
The document outlines the fundamentals of current Good Manufacturing Practice (cGMP) as presented by Md. Zakaria Faruki, emphasizing Silva Pharmaceuticals' commitment to quality, safety, and compliance with established guidelines. It covers the history of GMP, GxP guidelines, and the importance of maintaining high standards in pharmaceutical manufacturing to ensure safety, efficacy, and regulatory compliance. Additionally, it includes objectives, ten golden rules of GMP, and essential quality attributes for medicines.