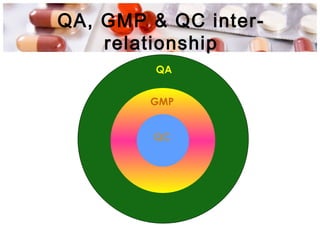
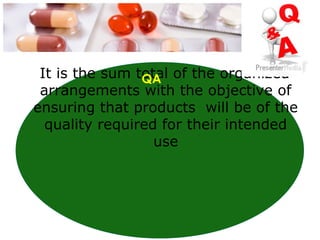
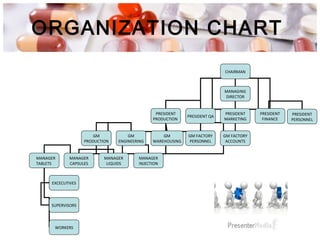
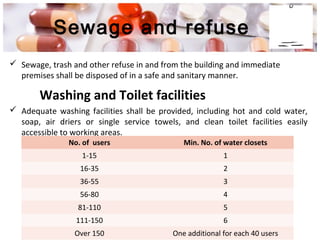
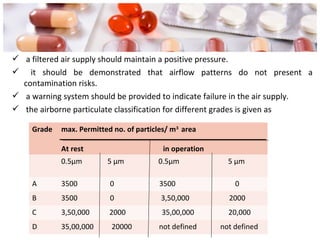
This document provides an overview of Good Manufacturing Practices (GMP) for pharmaceutical manufacturing. It defines GMP and explains that GMP aims to ensure consistent production of quality products through established processes and quality control. Key aspects of GMP covered include organization and personnel qualifications, facility and equipment design, material management, production operations, quality control testing, and documentation. Maintaining high standards of hygiene, sanitation, maintenance and training are emphasized. The goals of GMP are to minimize risks like contamination, incorrect dosing, and protect patient safety.