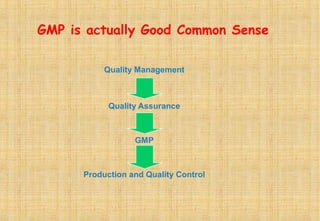
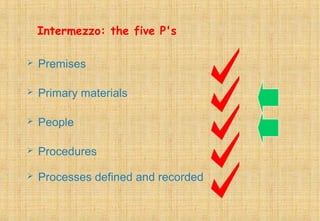
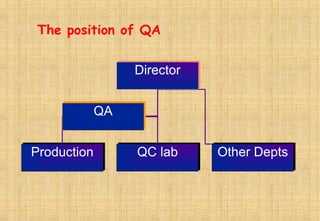
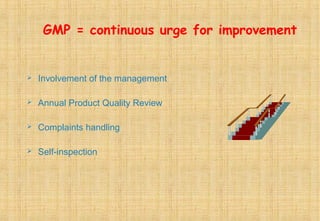
Good Manufacturing Practices (GMP) are essential for ensuring the quality and safety of drugs, with regulations established to guide manufacturers in maintaining high standards. Key principles of GMP include quality assurance, sanitation, personnel training, and systematic processes aimed at continuous improvement. Compliance with GMP is mandatory in regulated pharmaceutical markets and is crucial for effective quality management throughout the production lifecycle.