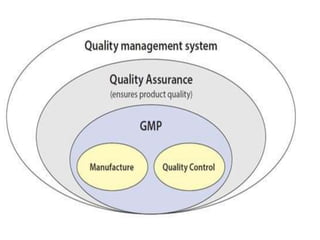
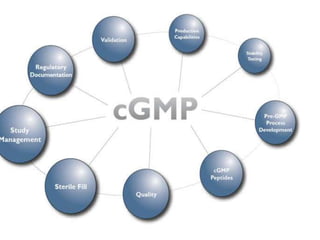
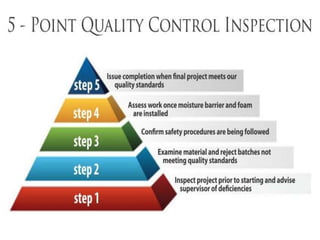
This document discusses Good Manufacturing Practices (GMP) and current Good Manufacturing Practices (cGMP). It provides definitions of GMP and cGMP, explaining that GMP ensures quality and safety in manufacturing while cGMP refers specifically to FDA regulations. The principles and regulations of GMP, cGMP, and their comparison are outlined. Key aspects like facilities, equipment, documentation, packaging and labeling, quality control, and standard operating procedures are summarized.