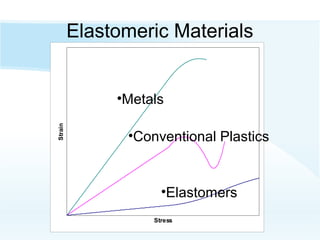
Elastomers are macromolecular materials known for their elasticity and viscoelastic properties, commonly found in materials like natural rubber and synthetic variants. They can be categorized into general-purpose, specialty, and thermoplastic elastomers, each with unique structures and properties that allow for extensive applications in various industries including automotive and electronics. While elastomers offer advantages such as recyclability and durability, they also have drawbacks including higher costs and limited heat resistance.