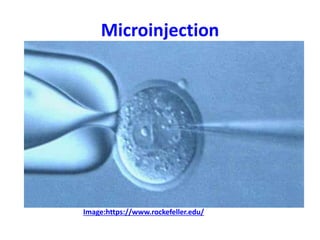
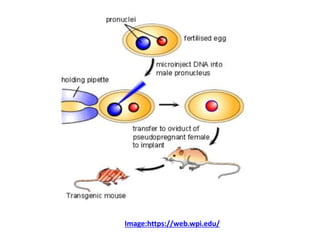
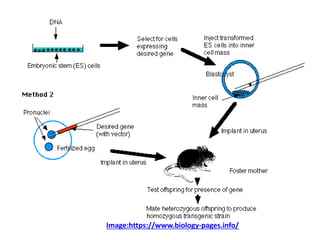
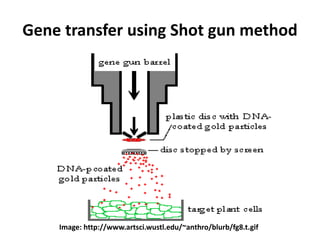
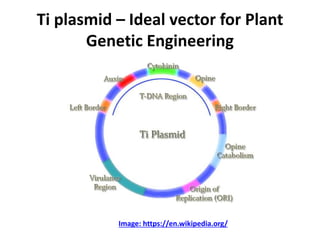
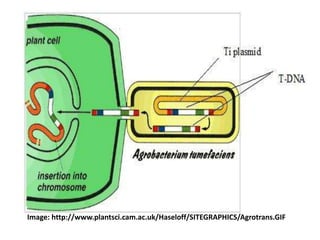
This document discusses various gene transfer techniques used in genetic engineering. It describes direct techniques like chemically stimulated DNA uptake using polyethylene glycol (PEG), transduction using bacteriophages, electroporation using high voltage electricity, and microinjection of DNA into fertilized eggs. It also discusses indirect techniques like microprojectile bombardment which shoots DNA-coated particles into plant cells, and Agrobacterium-mediated transfer where the bacterium transfers tumor-inducing (T-DNA) from its Ti plasmid into the host plant genome.