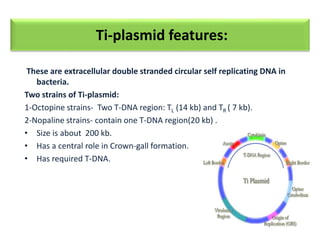
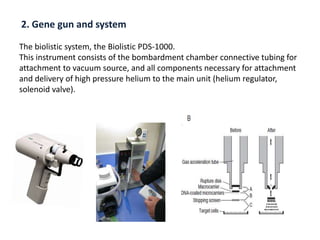
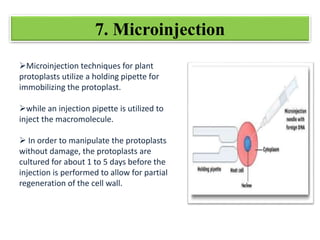
Genetic engineering involves manipulating an organism's genome using modern DNA technology. This document discusses various genetic transformation methods, including both direct and indirect methods. Indirect methods involve using Agrobacterium tumefaciens to transfer DNA into plant cells. Direct methods discussed include particle bombardment, polyethylene glycol treatment, electroporation, and microinjection. The document provides details on the process, mechanisms, applications and history of genetic engineering and transformation techniques.