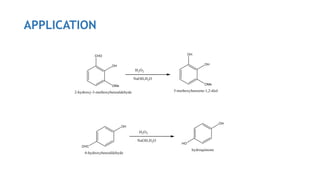
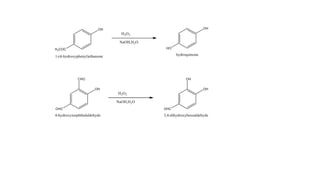
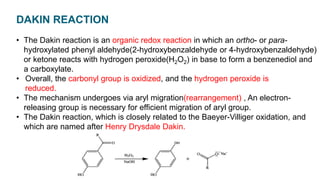
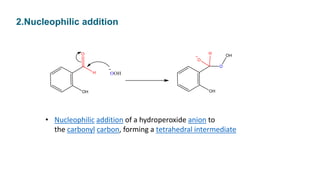
The Dakin reaction involves the oxidation of an ortho- or para-hydroxylated phenyl aldehyde or ketone with hydrogen peroxide in a basic solution. This results in the oxidation of the carbonyl group to a benzenediol and the formation of a carboxylate. The reaction proceeds through a nucleophilic addition, 1,2 aryl migration, hydrolysis, and phenoxide ion formation steps. The reactivity depends on factors like the electrophilicity of the carbonyl carbon and the speed of the 1,2 migration. Phenyl aldehydes react faster than ketones and ortho-hydroxy compounds faster than para-hydroxy derivatives in weak basic conditions. Electron donating groups increase reactivity while electron

![3. 1,2 Shift
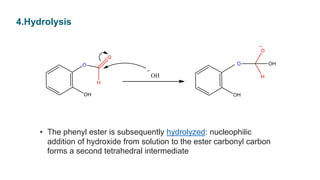
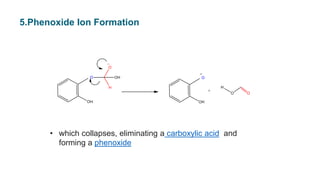
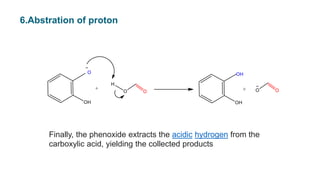
• The intermediate collapses,causing
[1,2] aryl migration, hydroxide elimination, and formation of a
phenyl ester](https://image.slidesharecdn.com/dakin-201022074923/85/Dakin-rearrangemnt-5-320.jpg)
![• The Dakin oxidation has two rate-limiting steps: nucleophilic addition of
hydroperoxide to the carbonyl carbon and [1,2]-aryl migration.
• Therefore, the overall rate of oxidation is dependent on the
nucleophilicity of hydroperoxide, the electrophilicity of the carbonyl
carbon, and the speed of [1,2]-aryl migration.
• The alkyl substituents on the carbonyl carbon, the relative positions of
the hydroxyl and carbonyl groups on the aryl ring, the presence of
other functional groups on the ring, and the reaction mixture pH are
four factors that affect these rate-limiting steps.
Factors affecting reaction kinetics](https://image.slidesharecdn.com/dakin-201022074923/85/Dakin-rearrangemnt-9-320.jpg)

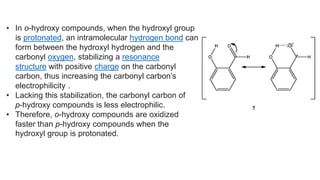
![Other functional groups on the aryl ring
• Substitution of phenyl hydrogens with electron-donating
groups ortho or para to the carbonyl group increases
electron density at the migrating carbon, promotes [1,2]-aryl
migration, and accelerates oxidation.
• Substitution with electron-donating groups meta to the
carbonyl group does not change electron density at the
migrating carbon; because unsubstituted phenyl group
migratory aptitude is low, hydrogen migration dominates.
• Substitution with electron-withdrawing
groups ortho or para to the carbonyl decreases electron
density at the migrating carbon, inhibits [1,2]-aryl migration,
and favors hydrogen migration](https://image.slidesharecdn.com/dakin-201022074923/85/Dakin-rearrangemnt-12-320.jpg)