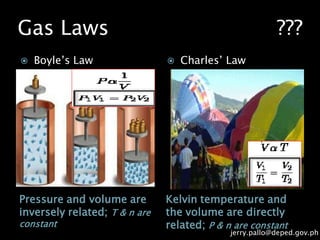
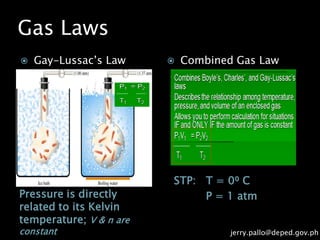
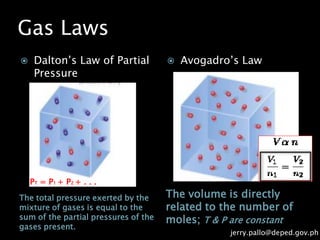
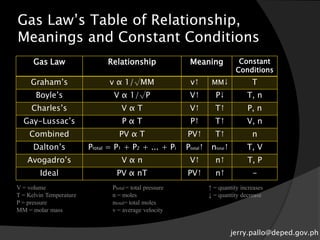
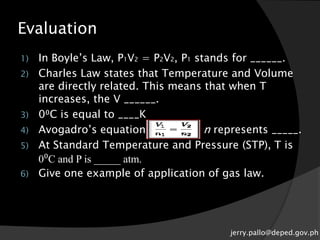
The document discusses gas laws and their applications. It outlines several gas laws including Boyle's law, Charles' law, Gay-Lussac's law, Dalton's law of partial pressures, and Avogadro's law. These laws describe the relationships between various gas properties including pressure, volume, temperature, and number of moles. The document also provides examples of how gas laws are applied in areas like bicycles, cars, and medical equipment. Students will demonstrate their understanding of gas behavior and laws through various performance tasks.