Embed presentation
Downloaded 128 times





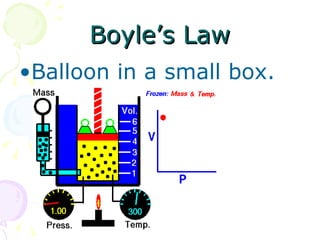


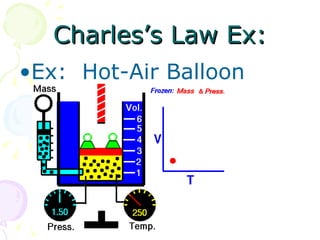

This document discusses key concepts relating to the behavior of gases, including the definitions of pressure and how it relates to force and area. It describes how pressure decreases with increasing altitude due to lower atmospheric pressure. Boyle's Law and Charles' Law are also summarized, with Boyle's Law stating that gas pressure increases as volume decreases if temperature remains constant, while Charles' Law indicates that gas temperature increases as volume increases if pressure remains constant. Charles' Law is used to calculate the temperature at which gas volume would reach absolute zero.









