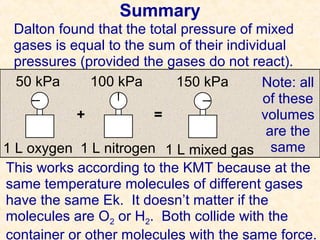
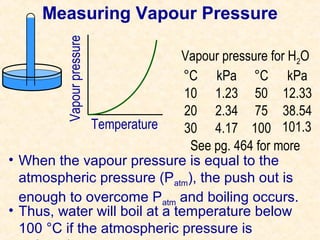
This document discusses gas laws and properties related to gas pressure. It begins with questions about gas pressure in balloons and containers filled with pure oxygen. It then summarizes Dalton's law of partial pressures, which states that the total pressure of a gas mixture is equal to the sum of the individual gas partial pressures. The document provides examples of calculating total and partial pressures. It continues discussing vapor pressure and how to measure and correct for vapor pressure when collecting gases over water. It concludes with sample calculations for determining the volume of dry gas collected at standard temperature and pressure.