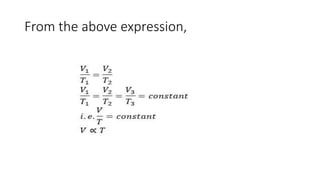
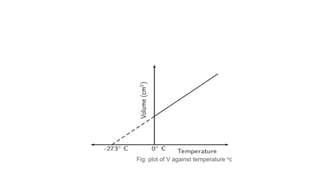
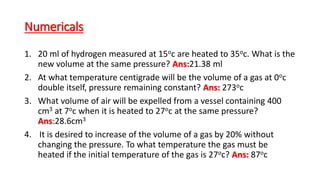
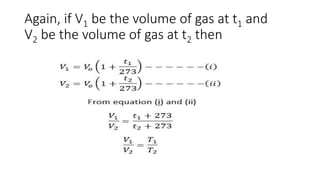Charle's law states that at a constant pressure, the volume of a gas increases or decreases by 1/273 of its original volume for each 1 degree Celsius increase or decrease in temperature. Specifically, the volume is directly proportional to the absolute temperature. This relationship has been verified experimentally by plotting graphs of volume versus temperature, which produce straight lines passing through the origin, indicating a direct proportionality. The law allows for calculations of gas volume changes with temperature changes and has applications like filling hot air balloons due to air's decreasing density when heated.