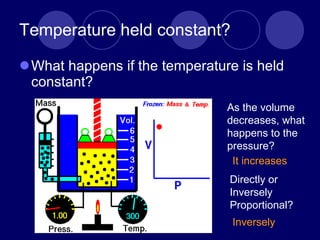
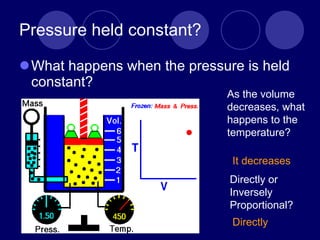
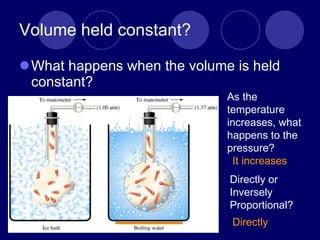
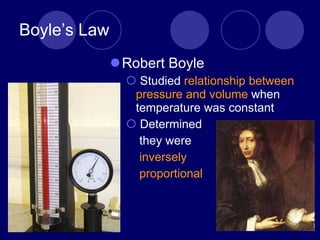
The document discusses the gas laws of Boyle's law, Charles' law, and Gay-Lussac's law. Boyle's law states that the pressure and volume of a gas are inversely proportional at constant temperature. Charles' law describes the direct proportionality of volume and temperature at constant pressure. Gay-Lussac's law found the direct relationship between pressure and temperature when volume is kept constant. The document also defines ideal gases as having no particle volume or intermolecular forces, while real gases do have particle volume and intermolecular attractive forces.