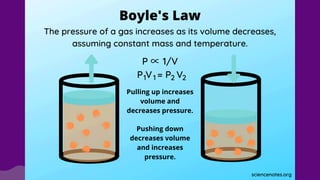
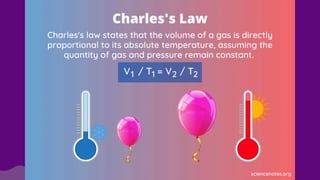
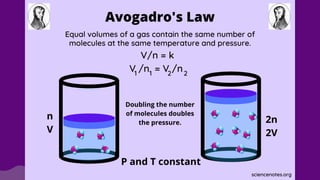
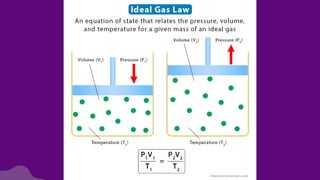
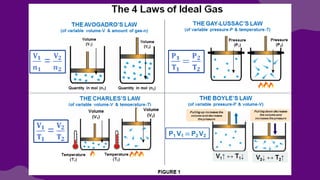
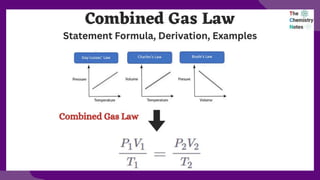
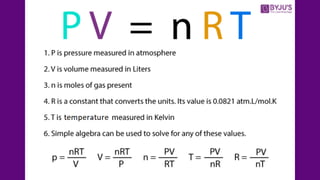
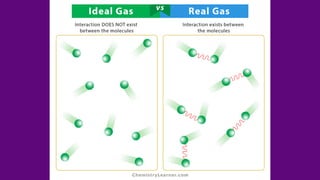
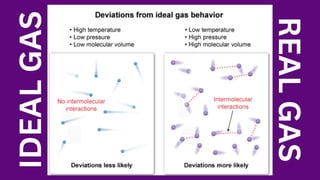
The document discusses gas laws, which describe the relationships between pressure, volume, temperature, and the amount of gas. It highlights three primary laws: Boyle’s Law, Charles’ Law, and Avogadro’s Law, along with the Ideal Gas Law that combines these principles. Additionally, it differentiates between ideal gases, which behave theoretically without interactions, and real gases, which exhibit volume and elastic collision characteristics due to intermolecular forces.