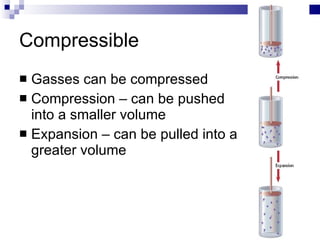
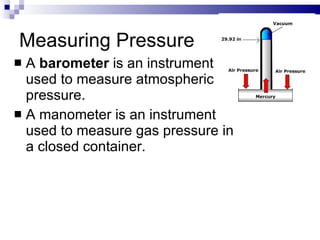
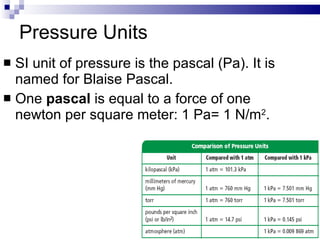
Kinetic Molecular Theory describes the behavior of gases in terms of particles in motion. It makes several assumptions about the size, motion, and energy of gas particles. Specifically, it assumes that gas particles are small, move randomly in straight lines until colliding elastically, and have a distribution of kinetic energies determined by their masses and velocities. This theory explains the low density, compressibility, and diffusion of gases as well as how temperature and pressure arise from the motion and collisions of gas particles.