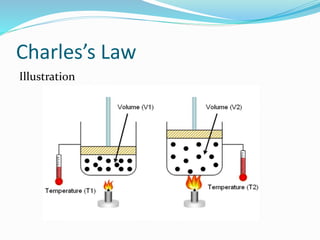
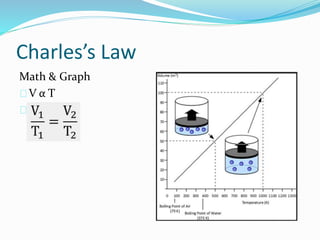
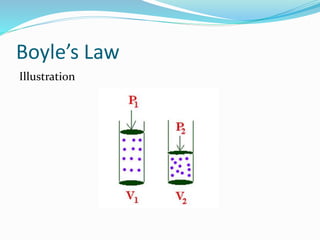
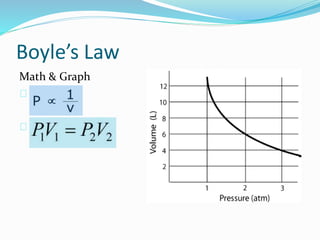
The document discusses Charles's Law and Boyle's Law. Charles's Law states that for an ideal gas at constant pressure, the volume is directly proportional to the temperature. Boyle's Law states that for a fixed amount of an ideal gas kept at a fixed temperature, volume and pressure are inversely proportional. The document then provides examples and demonstrations of these gas laws, followed by a scenario asking why a bicycle tire went flat again soon after being changed, likely related to the temperature changes described in Charles's Law.