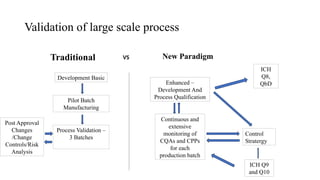
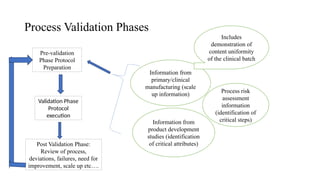
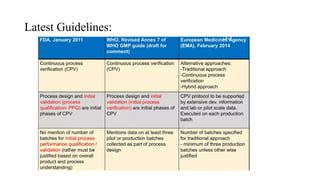
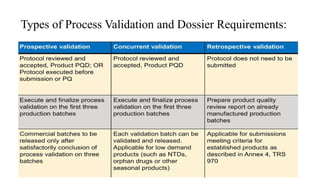
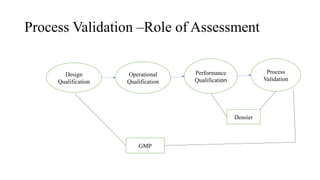
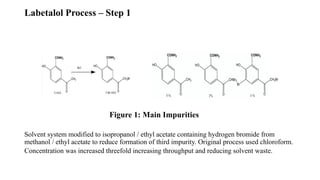
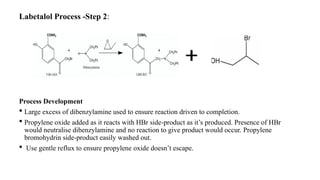
The document discusses the importance of process control in pharmaceutical process chemistry, which ensures product quality, safety, and regulatory compliance through monitoring critical process parameters and employing techniques such as process analytical technology and quality by design. It elaborates on validation of large-scale processes, including phases like design, operational, and performance qualifications, while highlighting case studies like labetalol production to illustrate practical applications. Overall, it emphasizes a systematic approach to manufacturing that integrates scientific principles and advanced technologies to produce effective pharmaceutical products.