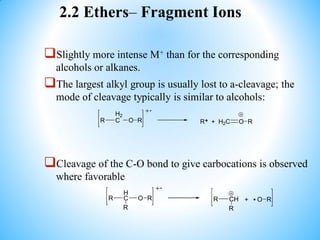
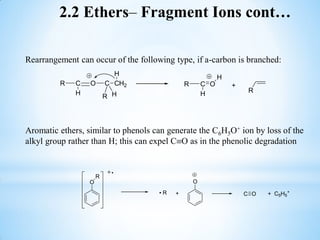
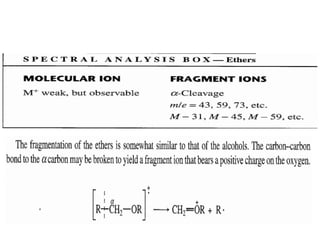
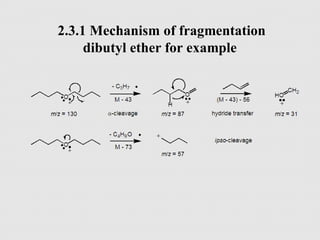
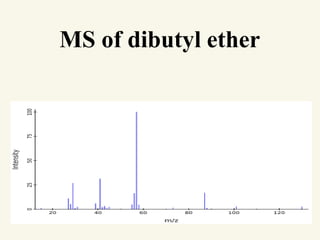
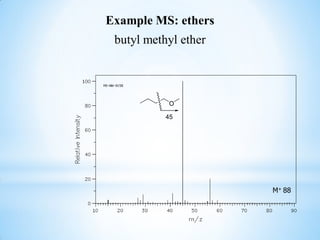
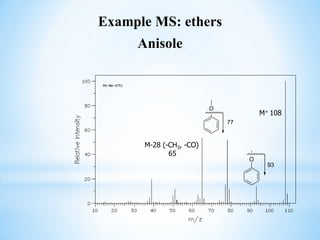
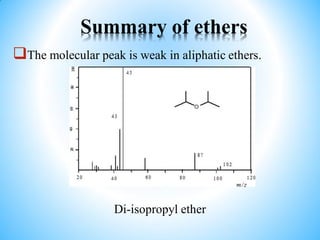
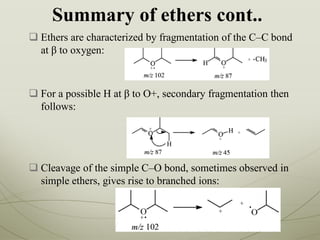
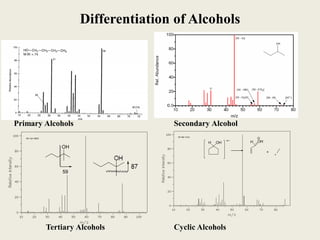
This document outlines a graduate program on mass spectral analysis of alcohols, phenols, and ethers. It discusses the objectives to introduce mass spectroscopy, principles, functions, ionization techniques, and fragmentation patterns of various compounds. Specifically, it describes how primary, secondary, tertiary, and cyclic alcohols can be differentiated by their mass spectrometry peaks. It also examines the fragmentation of aromatic alcohols, phenols, and various types of ethers including aliphatic and aromatic ethers. The summary provides key differences in molecular ion peaks and fragmentation patterns between these compound classes.







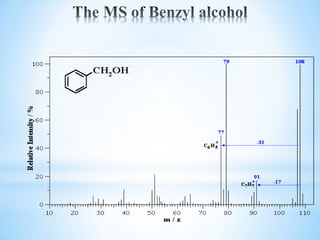
![1.6 Alcohols
General properties of alcohols in mass spectroscopy:
The molecular ion is usually small and sometimes
undetectable especially in tertiary alcohols.
In primary and secondary alcohols, the identification
of molecular ion is complicated by the prevalence of
a [M-1] peak caused by the loss of single hydrogen
from the alpha carbon.
The compound can identified as an alcohol by the
presence of the [M-H], [M-OH] and m/z 31 that are
all characteristic of alcohol.](https://image.slidesharecdn.com/mscalcoholsphenolsethers-190817075348/85/Msc-alcohols-phenols-ethers-13-320.jpg)
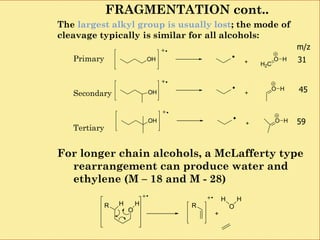
![1.6.1 Primary Alcohols
Can be identified as an alcohol because of the
characteristic at [M-H2O], M-18] and M/z 31.
The peak at m/z 31 can attributed to primary
alcohol because it is one of the larger peak in
the spectrum.
Primary alcohol show a peak resulting from
m/z 31.](https://image.slidesharecdn.com/mscalcoholsphenolsethers-190817075348/85/Msc-alcohols-phenols-ethers-14-320.jpg)
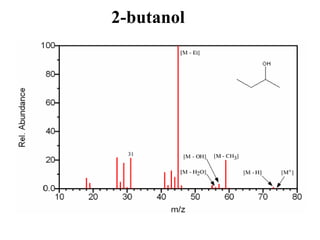
![1.6.2 Secondary Alcohol
the small peak at m/z 31 indicate that this
alcohol is not a primary alcohol.
The presence of a [M-et] and [M-CH3] peak
indicates that is the secondary alcohol.
Secondary alcohol show a peak resulting from
{m/s 45,59,73 and etc}.](https://image.slidesharecdn.com/mscalcoholsphenolsethers-190817075348/85/Msc-alcohols-phenols-ethers-16-320.jpg)
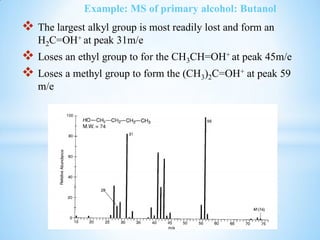
![Example: MS of Secondary alcohol:
This figure below must contain an even
molecular ion since the major peaks (m/z 59 and
45 are both odd).The compound can be identified
as an alcohol by the presence of the [M – H], [M
– OH], and m/z 31 peaks that are all
characteristic of alcohols. The small peak at m/z
31 indicates that this alcohol is not a primary
alcohol. The presence of a [M – Et] and [M –
CH3] peak indicates that this four carbon alcohol
(determined from its molecular mass) is the
secondary alcohol 2-butanol.](https://image.slidesharecdn.com/mscalcoholsphenolsethers-190817075348/85/Msc-alcohols-phenols-ethers-17-320.jpg)

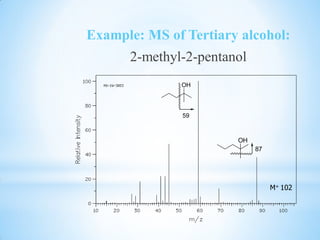
![1.6.4 Cyclic Alcohols
o Cyclic alcohols fragment similar to striaght
chain alcohols in that they give a [M-1] peak
from the loss of hydrogen and [M-18] peak
from the loss of water.
o They also create a peak at m/z 57 via a
complex ring cleavage.](https://image.slidesharecdn.com/mscalcoholsphenolsethers-190817075348/85/Msc-alcohols-phenols-ethers-21-320.jpg)
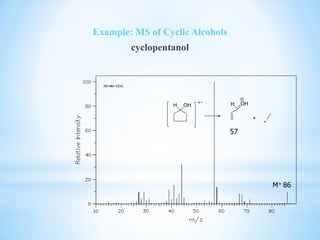


![1.8 Mass spectral analysis of Phenols
Phenols usually give a weak peak at m/z 77 attributed to a
rearrangement and can be identified by to peaks at [M-CO] and [M-
COH]. Example MS: Phenols
Phenol
-CO 66
-HCO 65 M+ 94](https://image.slidesharecdn.com/mscalcoholsphenolsethers-190817075348/85/Msc-alcohols-phenols-ethers-28-320.jpg)