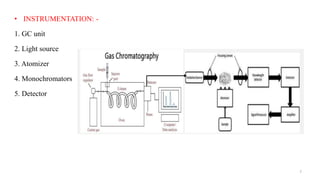
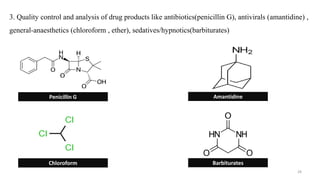
This document discusses gas chromatography-atomic absorption spectroscopy (GC-AAS), a hyphenated technique that combines gas chromatography and atomic absorption spectroscopy. GC-AAS allows for the separation and elemental analysis of sample components. The document outlines the basic components and workings of GC and AAS individually, and how they are interfaced to perform GC-AAS. Some key applications of GC-AAS mentioned include analysis of drugs, metabolites, pollutants, and impurities in various samples.