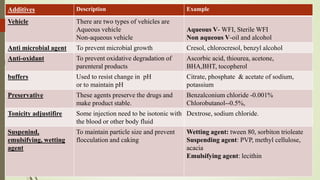
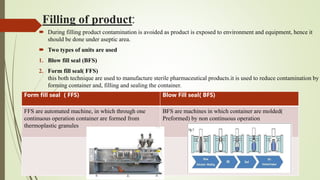
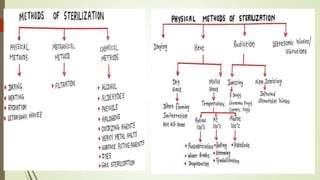
This document summarizes the key aspects of sterile manufacture of parenteral products. It describes the types of parenteral preparations, facility requirements including layout and laminar air flow, additives used in formulations, production planning such as cleaning, filling, and sealing processes. It also discusses the quality control tests including sterility, pyrogen, clarity, and leakage tests to ensure the sterile products are free of microorganisms and safe for patient use.