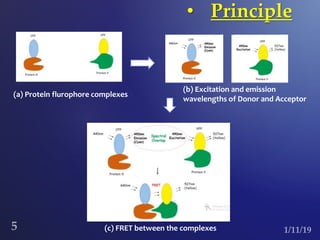
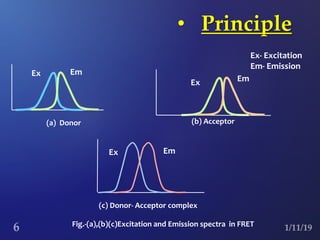
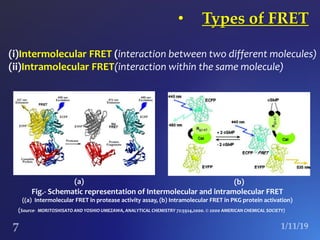
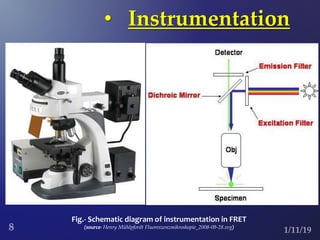
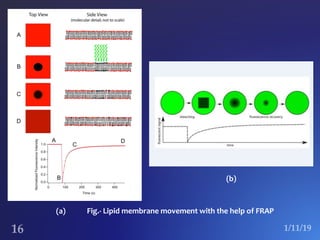
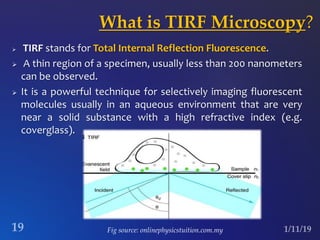
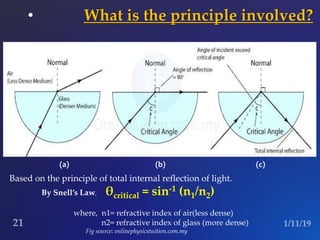
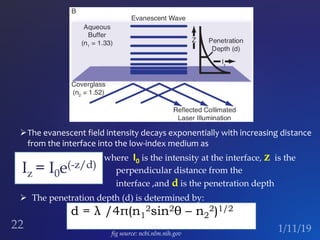
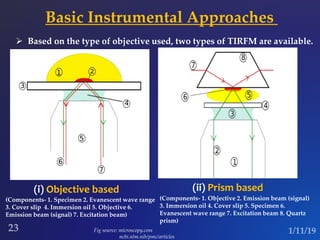
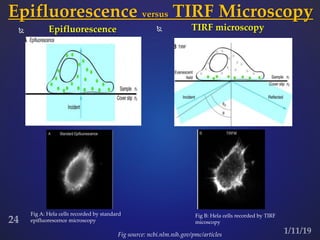
The document discusses three fluorescence microscopy techniques: FRET, FRAP, and TIRF microscopy. It explains the principles, instrumentation, sample preparation, applications, and future aspects of each technique. FRET involves energy transfer between fluorophores and is used to study molecular interactions and structure. FRAP examines fluorophore diffusion by photobleaching a region and monitoring recovery. It provides information about molecular mobility. TIRF microscopy uses evanescent waves to selectively excite fluorophores very near a surface and is applied to studies of cellular processes at membranes. All three techniques provide insights into biological phenomena at the molecular level.