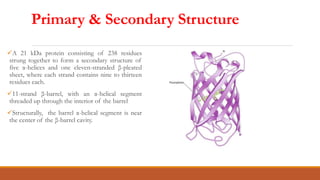
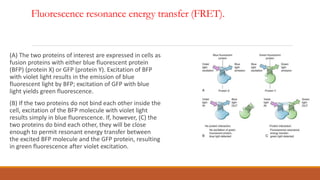
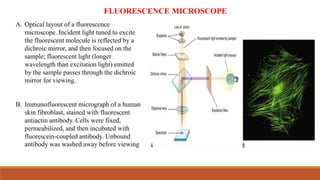
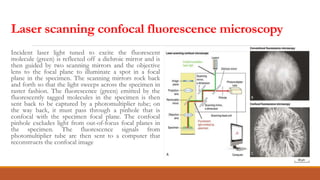
Green fluorescent protein (GFP) is a protein isolated from jellyfish that emits green light when exposed to blue light. It consists of 238 amino acid residues arranged in a beta-barrel structure with an alpha-helix running through the center. The fluorophore of GFP is formed by three amino acids in the central alpha-helix. GFP has become an important research tool, used as a marker for gene expression, protein localization and interactions by fusing it to genes or proteins of interest. It allows non-invasive visualization of biological processes using fluorescence microscopy.