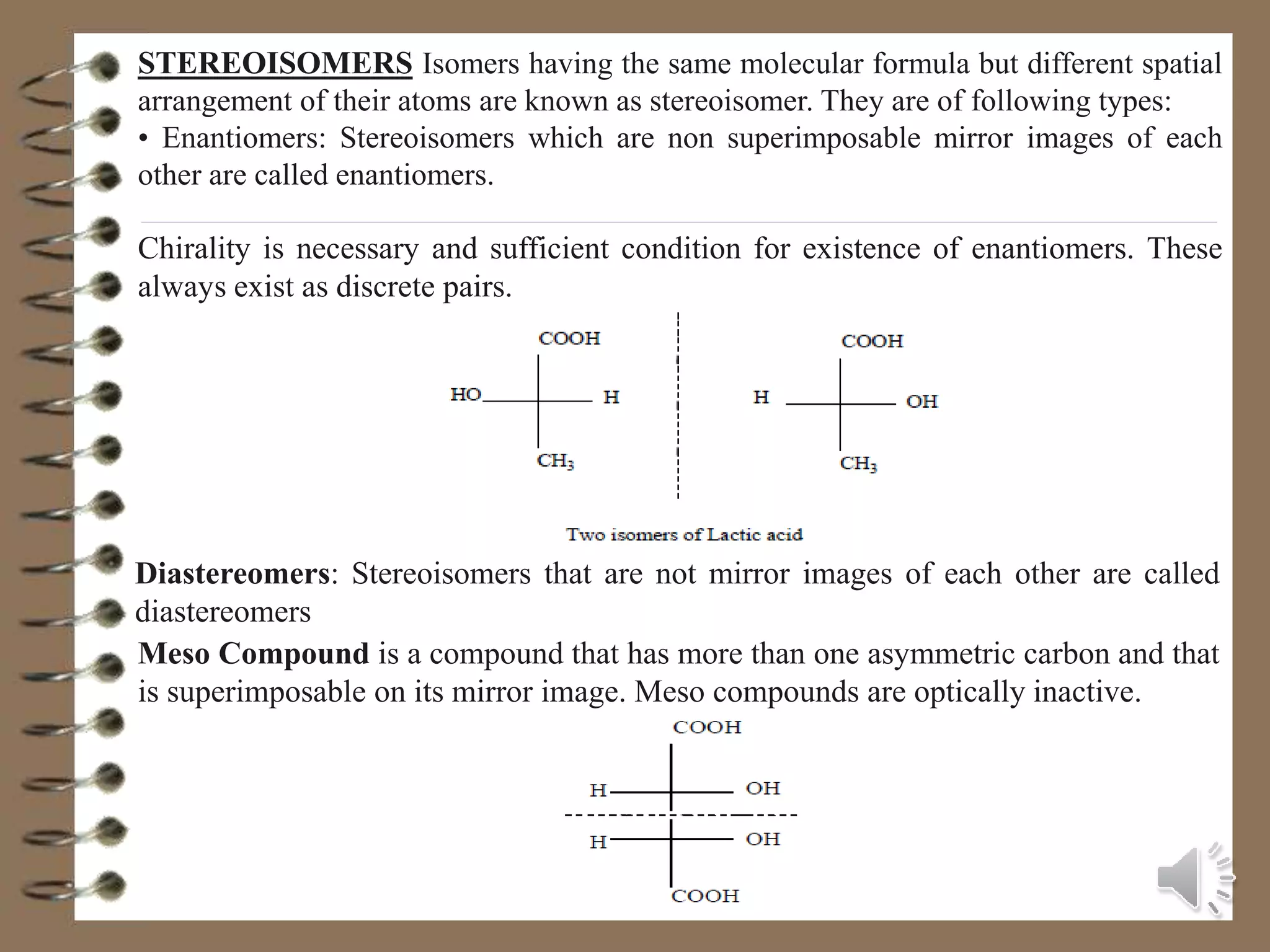
The document discusses various topics in stereochemistry including:
1. Stereochemistry deals with the three dimensional structure of molecules and how it affects properties.
2. Isomers can be classified as structural, positional, functional, ring-chain, metamers, and tautomeric depending on their structure.
3. Chiral molecules have "handedness" and lack a plane of symmetry, allowing the existence of enantiomers which are non-superimposable mirror images. Systems like R/S, D/L, and E/Z are used to describe chiral molecule stereochemistry.