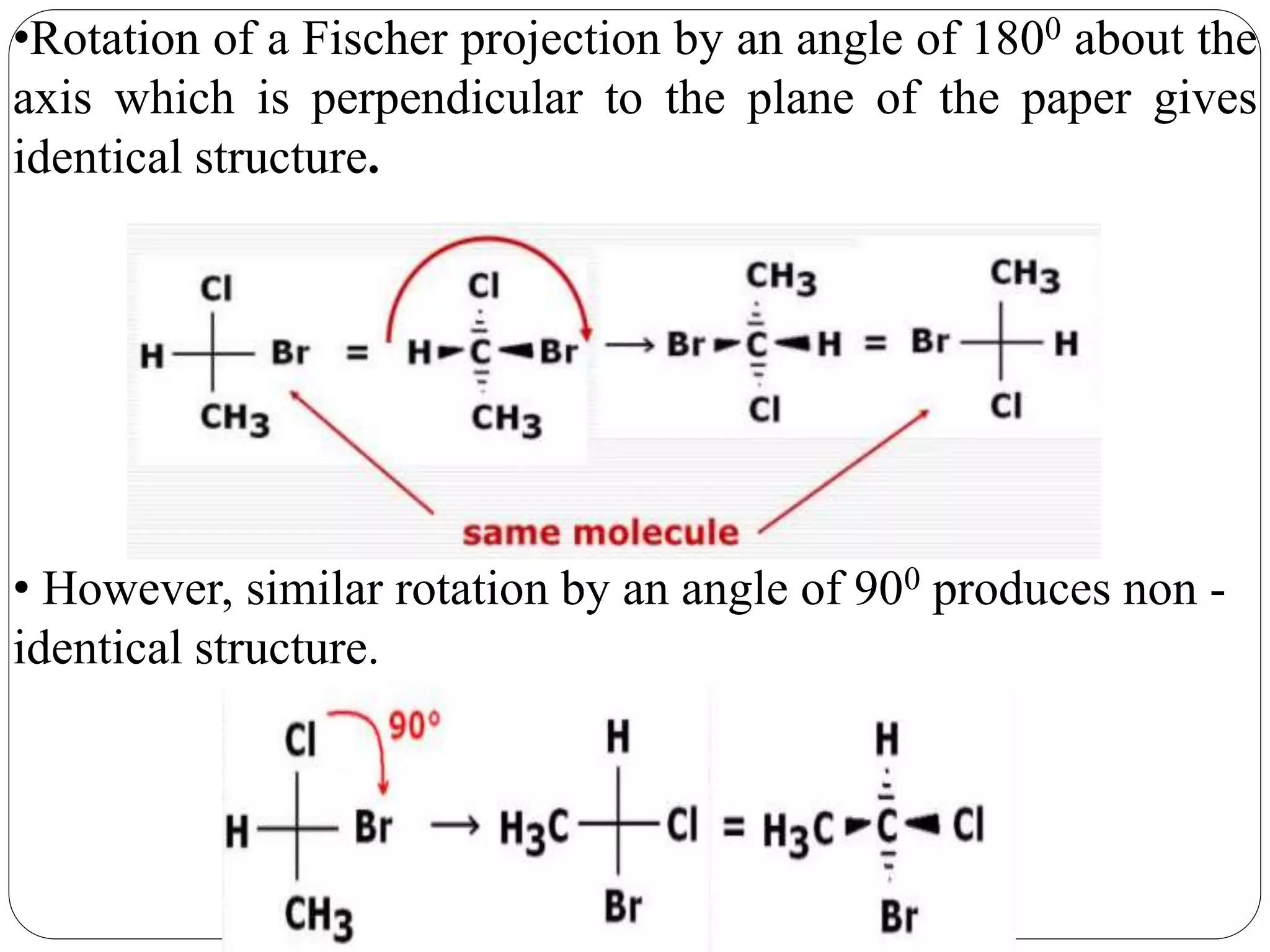
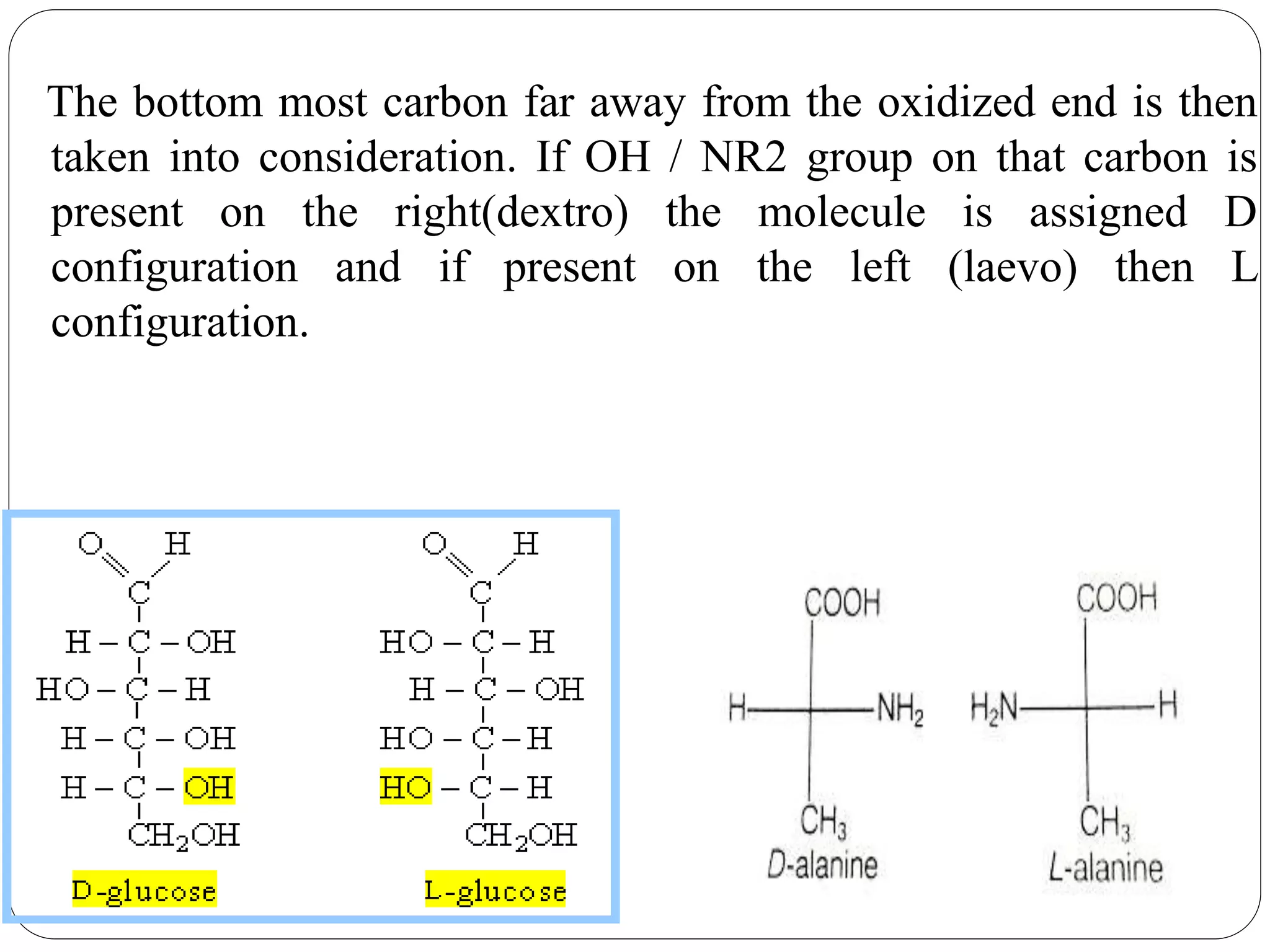
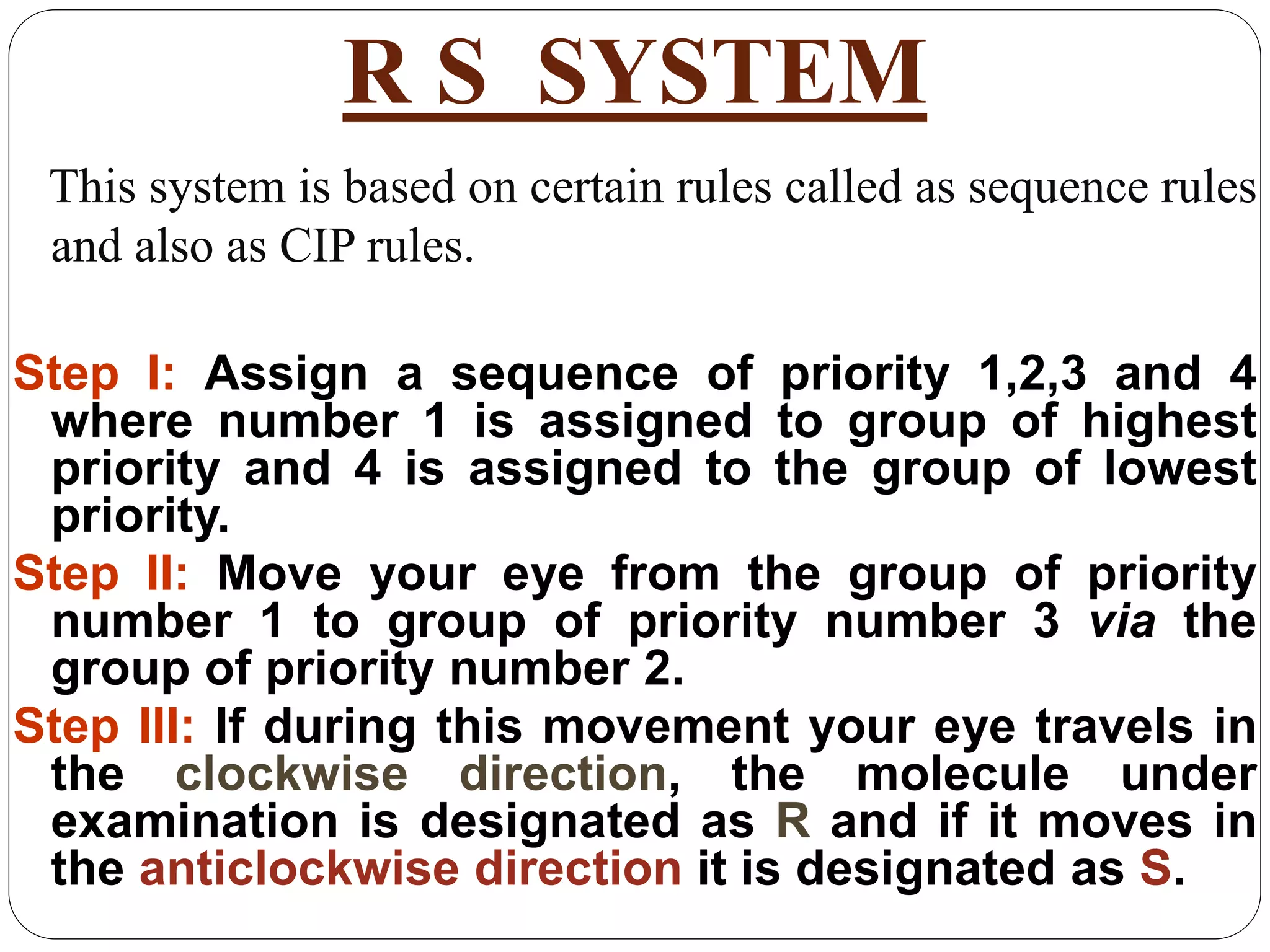
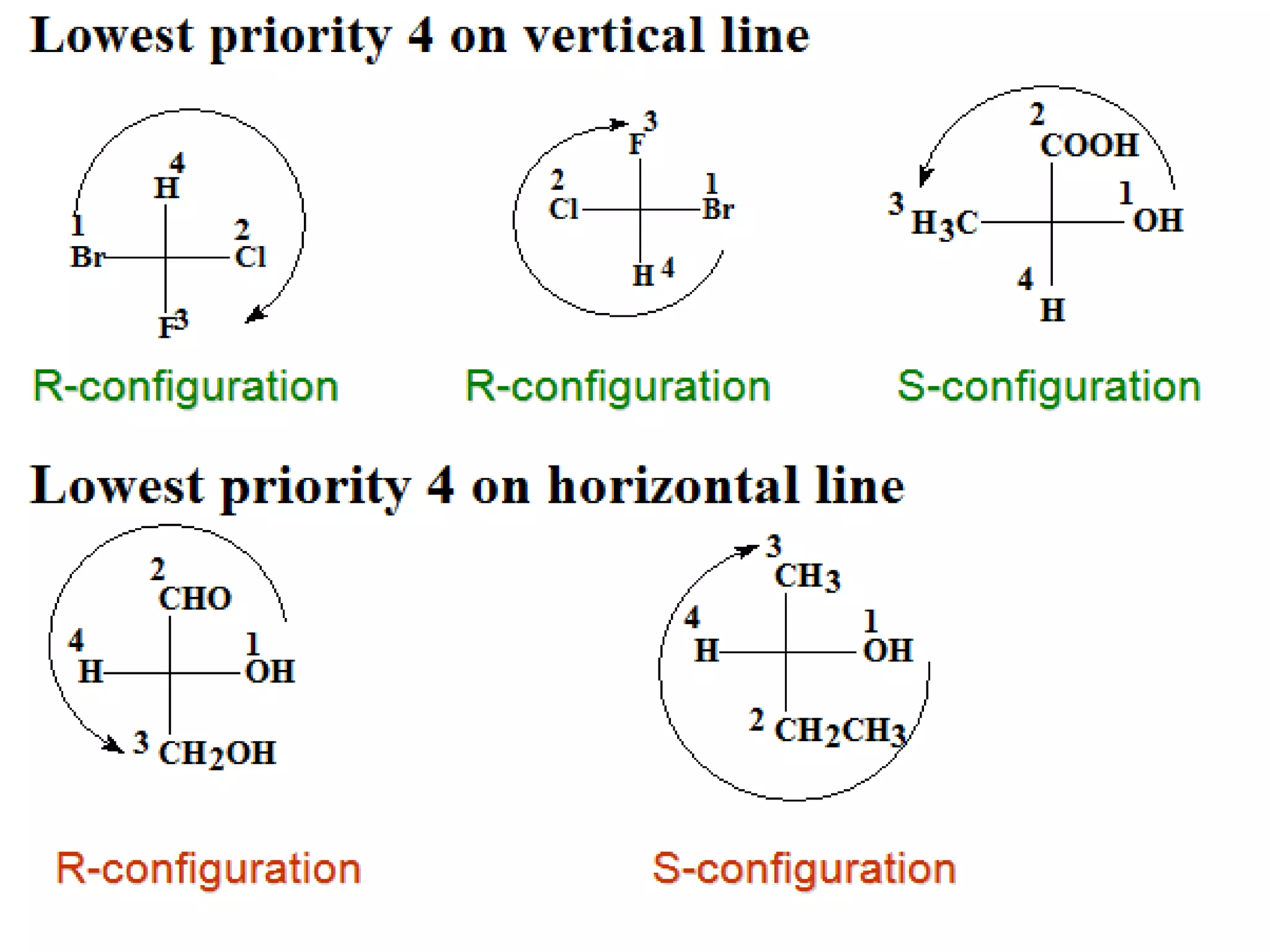
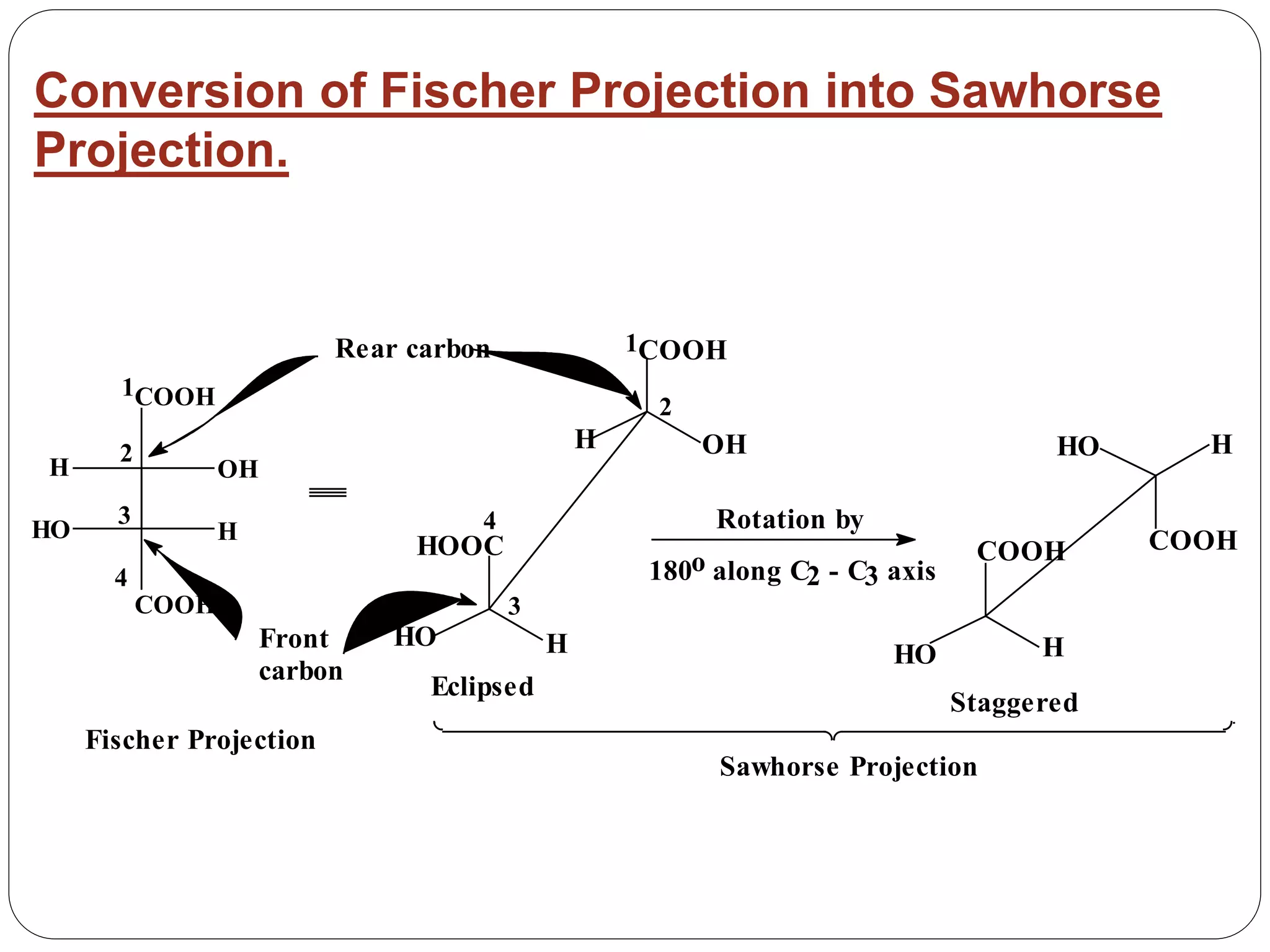
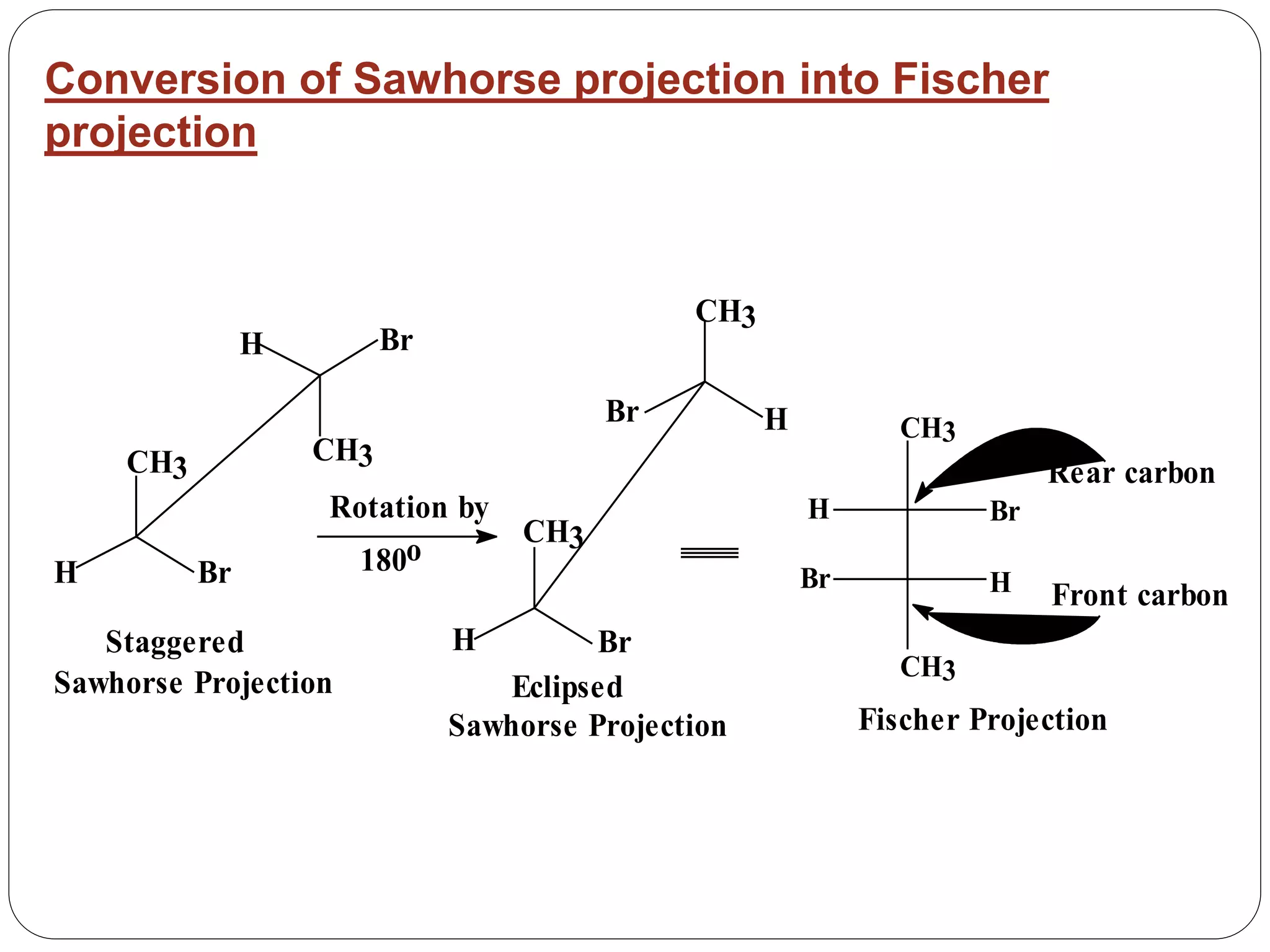
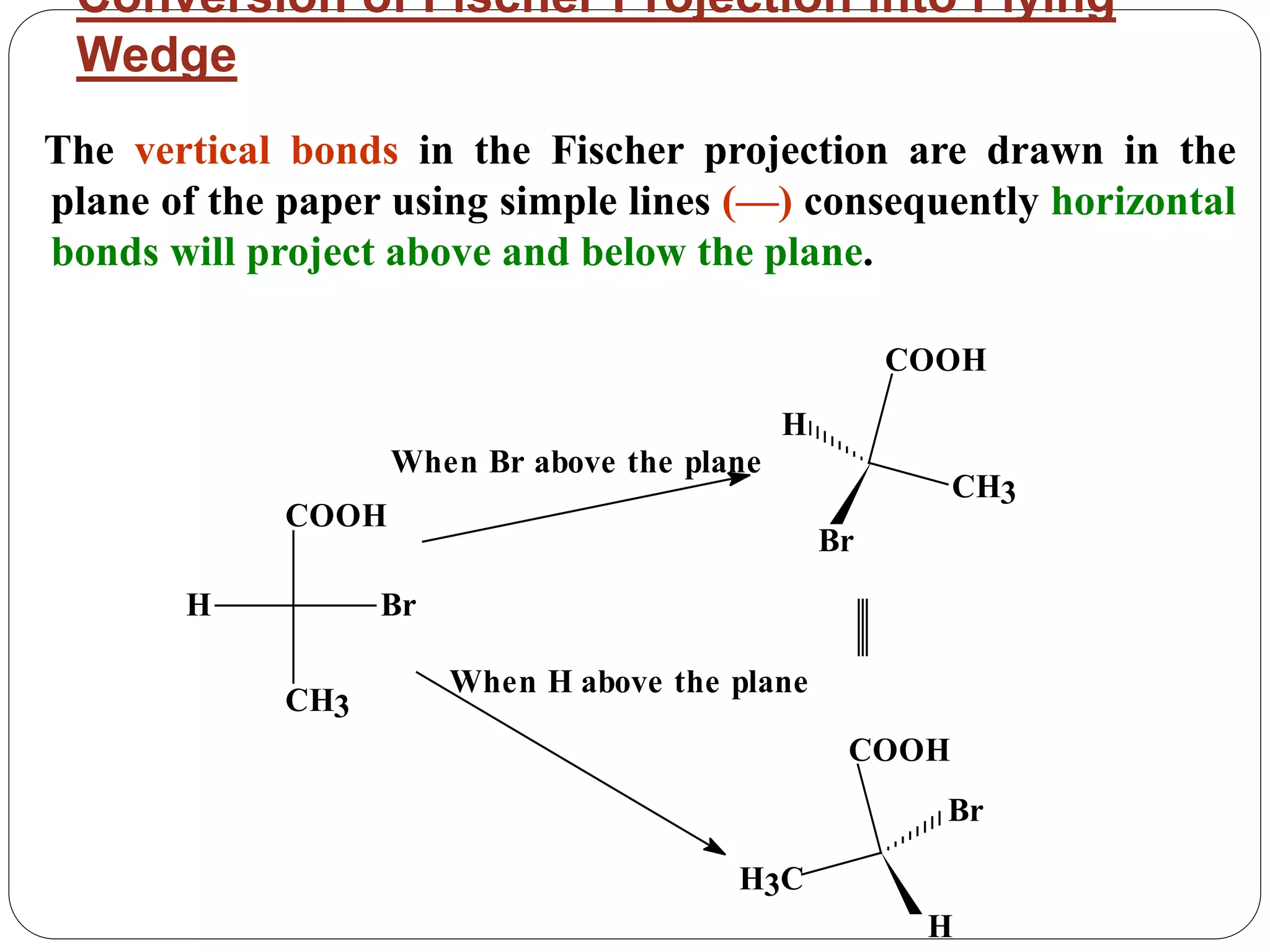
The document explains various stereochemical formulae used to represent three-dimensional molecular structures, including flying wedge, Newman, sawhorse, and Fischer projections. Each method highlights different aspects of molecular bonding and spatial arrangement, particularly for chiral centers. It also discusses the conversion between these projection types and the R/S nomenclature for chirality assignment.