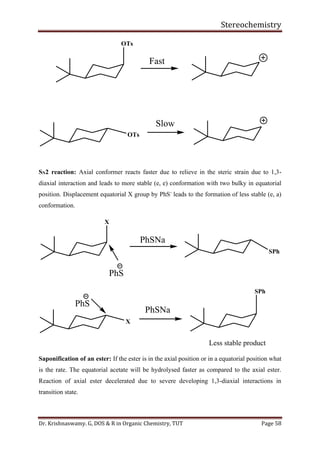
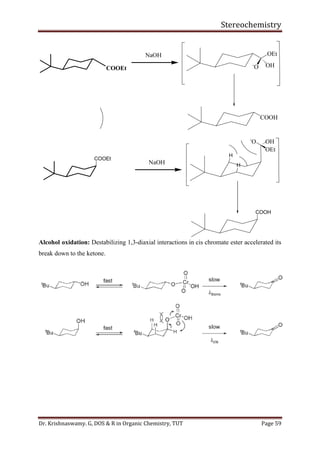
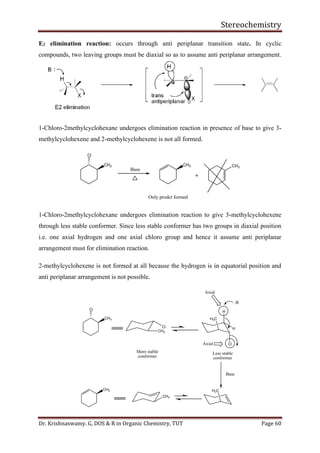
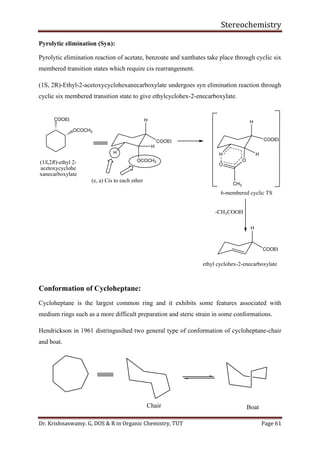
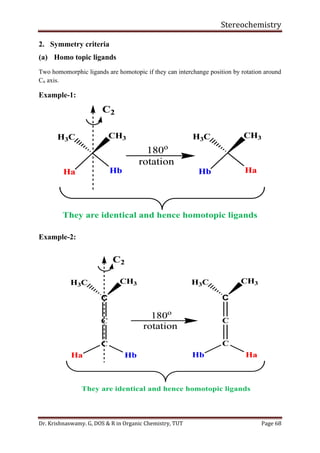
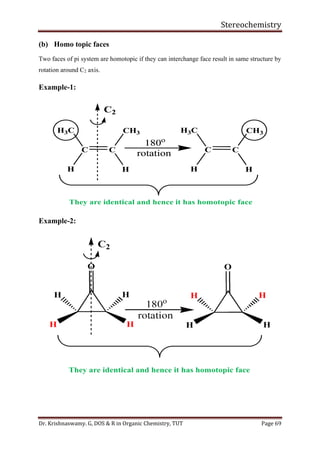
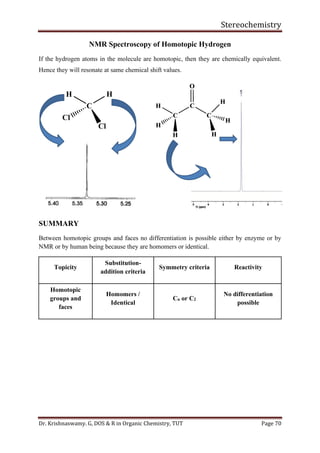
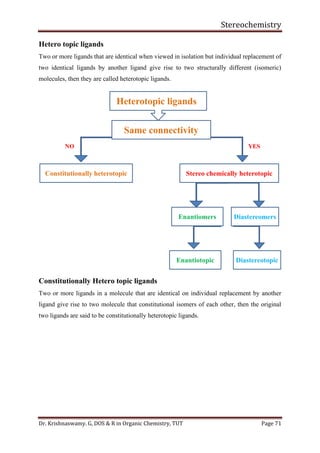
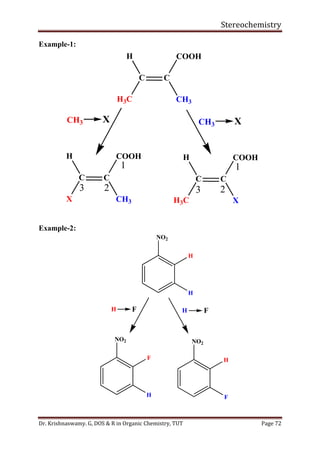
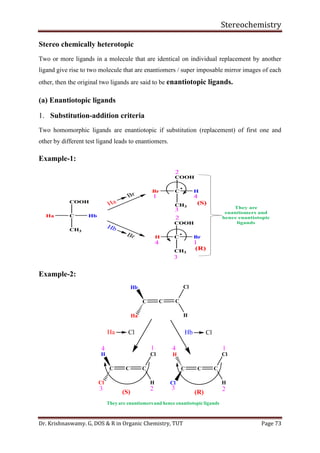
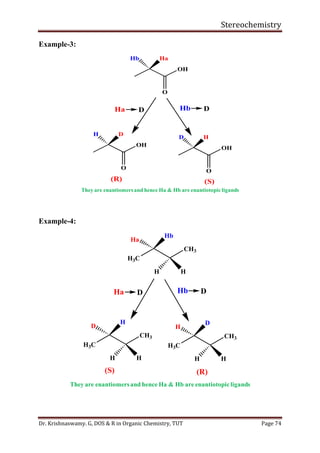
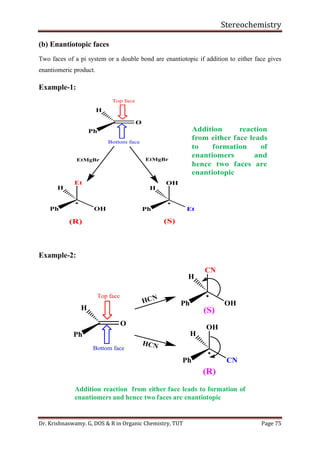
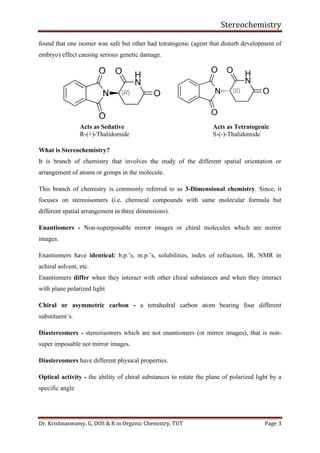
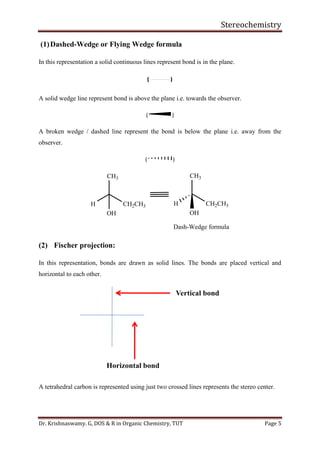
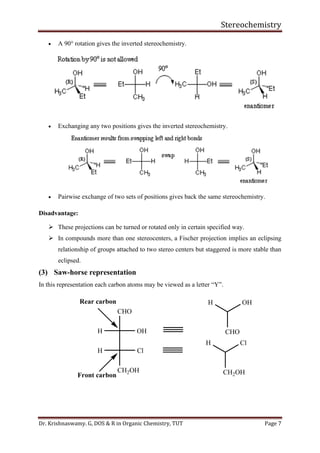
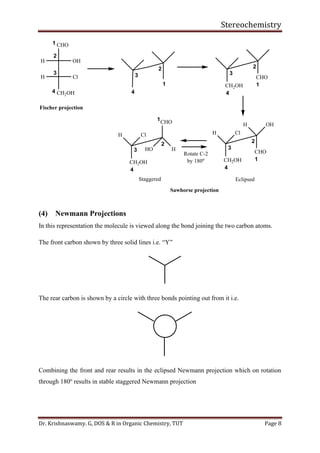
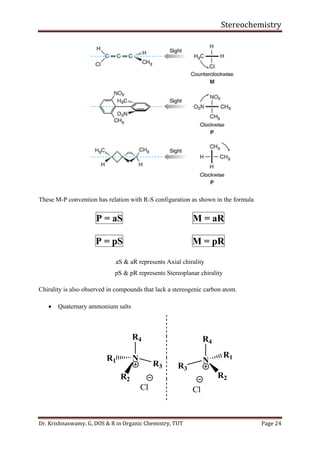
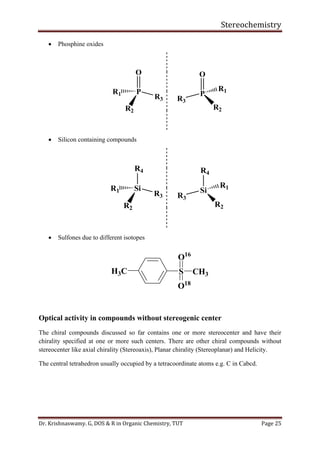
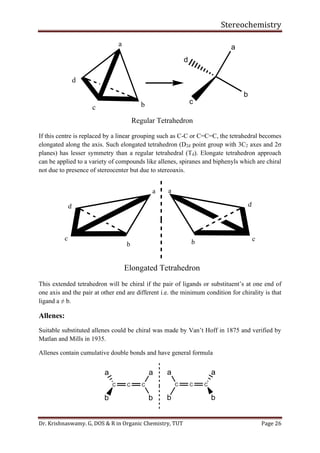
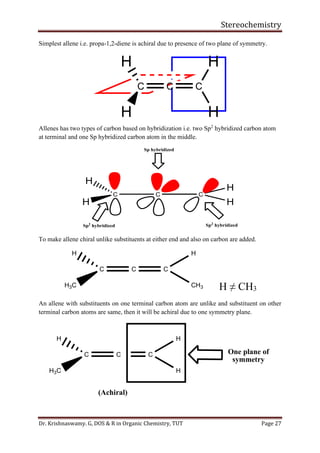
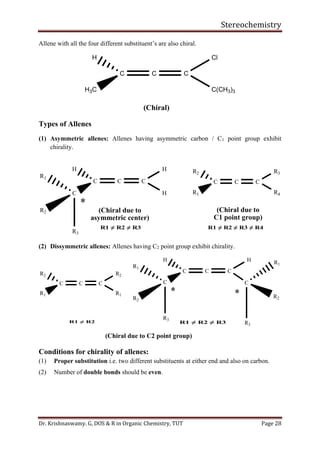
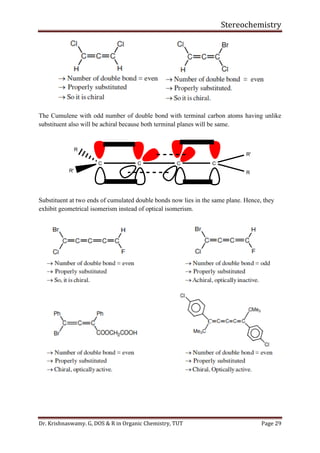
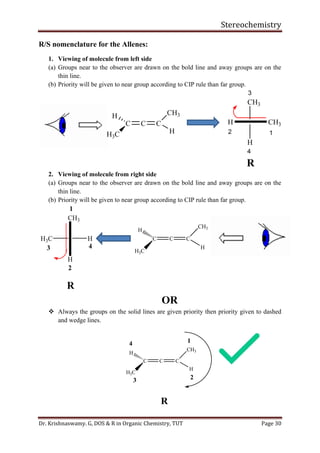
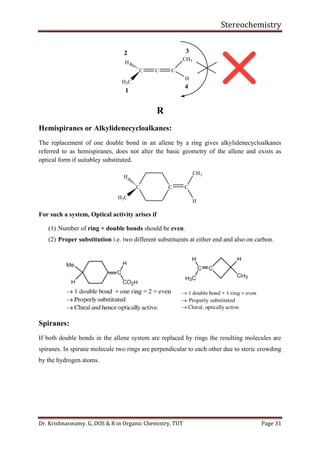
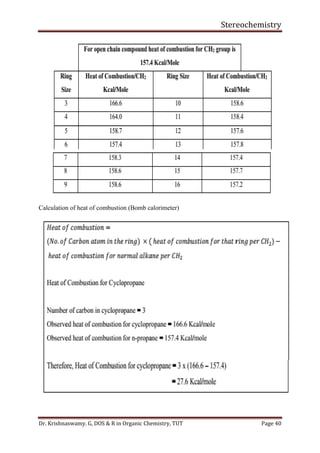
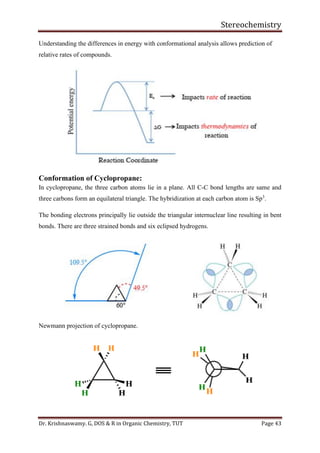
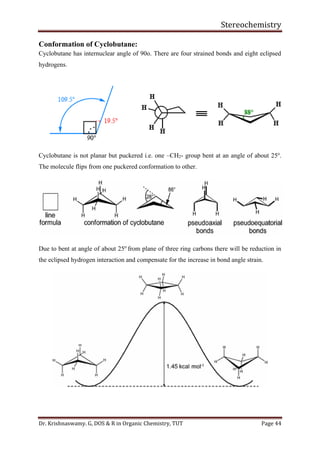
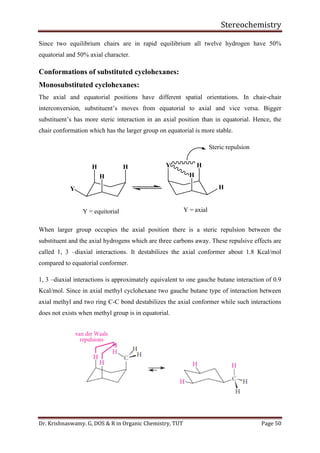
This document provides a comprehensive overview of stereochemistry, including its history, fundamental concepts, and various representations of chiral molecules. Key topics covered include the significance of stereochemistry in drug safety, types of isomers like enantiomers and diastereomers, and methods for determining configurations such as the R-S system. Visual representations and conversion methods for different stereochemical projections are also discussed, highlighting the complexity and importance of three-dimensional arrangements in organic chemistry.





















![Stereochemistry
Dr. Krishnaswamy. G, DOS & R in Organic Chemistry, TUT Page 52
H
H
C
H3C
H
H
H
H
C
H
H
CH3
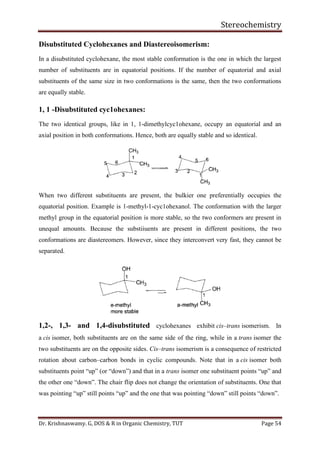
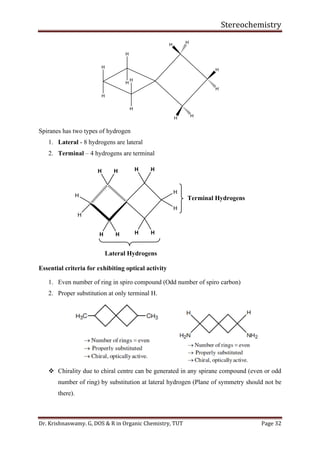
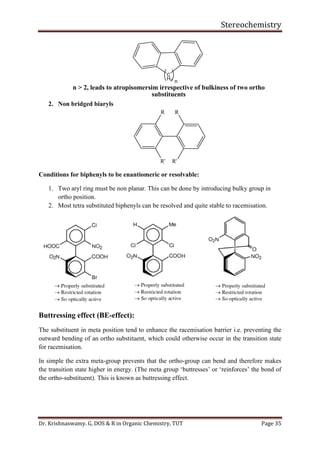
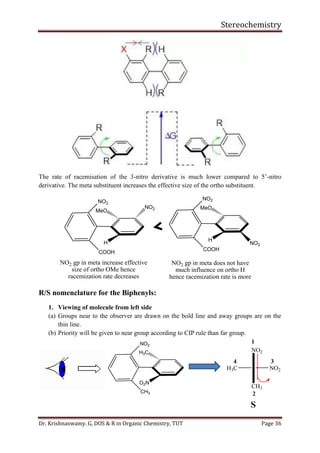
With isopropyl substituent, can still have a conformer with hydrogen pointed towards 1, 3-
hydrogens. Has energy of about 2.1 Kcal/mol.
H
H
C
H3C
H
CH3
H
H
C
H
CH3
CH3
With tertiary butyl group must have methyl group towards 1, 3-hydrogens. Its energy is
greater than 4.5 Kcal/mol.
H
H
C
H3C
CH3
CH3
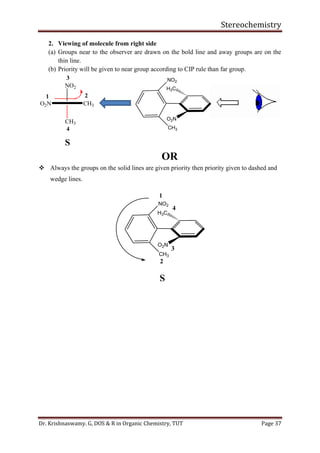
The relative population of the two chair conformers can be calculated by the equation,
The free-energy difference between conformers is referred to as the conformational free
energy. For substituted cyclohexanes it is conventional to specify the value of -Gc for the
equilibrium:
[Axial][Equatorial]](https://image.slidesharecdn.com/stereochemistrynotes-191118155630/85/Stereochemistry-notes-52-320.jpg)