This document provides an overview of stereochemistry concepts including:
- 3D representations like wedge-dash, Fischer, Newman, and sawhorse projections
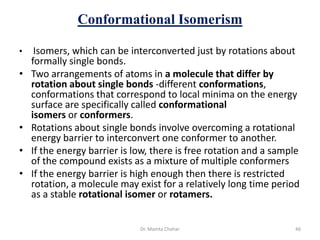
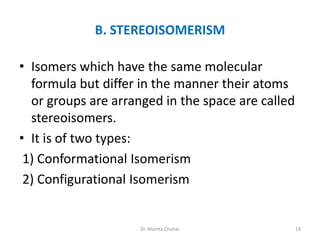
- Isomerism including structural, stereoisomerism (conformational and configurational), and optical activity
- Configurational isomers like enantiomers, which are non-superimposable mirror images that rotate plane-polarized light in opposite directions, and diastereomers, which are not mirror images
- Chirality, symmetry, resolution of racemic mixtures, meso compounds, and the Cahn-Ingold-Prelog system of assigning absolute configuration























![The formula for determining the number of stereoisomers as follows:
a) When the molecule is unsymmetrical and contains ''n '' chiral
carbon atoms,
Total no. of stereoisomers = 2n
b) When the molecule is unsymmetrical and has even number of
stereogenic centres or chiral carbon atoms,
Total no. of stereoisomers = No. of optical isomers + No. of meso forms
= 2(n-1) + 2(n/2-1)
c) When the molecule is symmetrical and has odd no. of stereogenic
centres,
Total no. of stereoisomers = [ 2(n-1)-2(n/2-1/2)]
= [(optical active isomers)+ 2(n/2-1/2) (meso- form)]
STEREOISOMERS
25
Dr. Mamta Chahar](https://image.slidesharecdn.com/steriochemistryppt29march2020-210429101405/85/Steriochemistry_MAMTA-25-320.jpg)






![• When light passes through a sample that can rotate plane polarised
light, the light appears to dim because it no longer passes straight
through the polarising filters. The amount of rotation is quantified
as the number of degrees that the analysing lens must be rotated
by so that it appears as if no dimming of the light has occurred.
Measuring Optical Activity
• When rotation is quantified using a polarimeter it is known as
an observed rotation, because rotation is affected by path length (l)
and concentration (c,). When these effects are eliminated a
standard for comparison of all molecules is obtained, the specific
rotation, [α].
[α]λ
T = 100 θ / c.l
When,
c= concentration is expressed as g sample /100ml solution
l = path length travels through a sample
θ = how much of the sample is present that will rotate the light
Specific rotation is a physical property like the boiling point of a
sample.
33
Dr. Mamta Chahar](https://image.slidesharecdn.com/steriochemistryppt29march2020-210429101405/85/Steriochemistry_MAMTA-32-320.jpg)