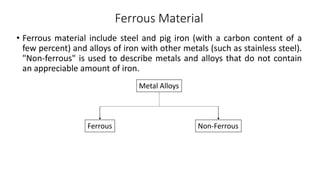
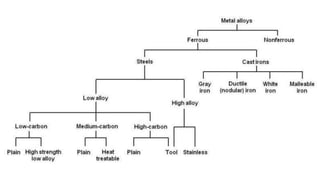
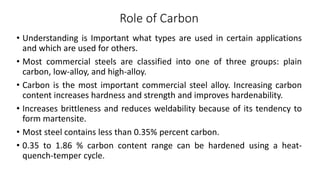
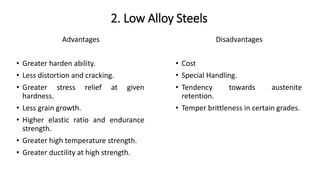
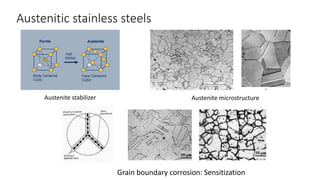
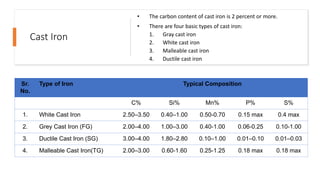
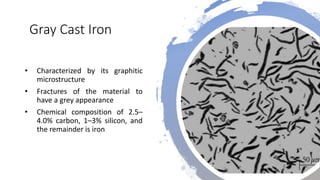
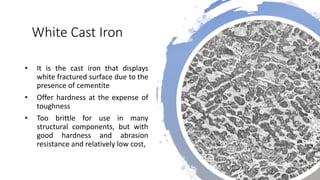
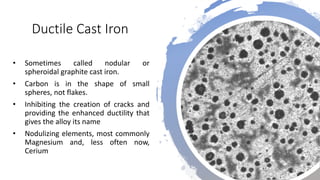
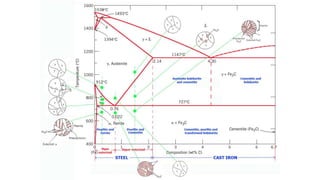
The document details the characteristics, properties, and classifications of ferrous and non-ferrous materials, particularly various types of steel and cast iron. It discusses the role of carbon and other alloying elements in affecting the mechanical properties of these materials, such as hardness, strength, and weldability. Additionally, it highlights specific applications for different steel classifications, including low carbon, medium carbon, high carbon, low alloy, and high alloy steels, along with different types of cast iron.