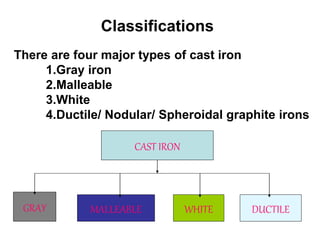
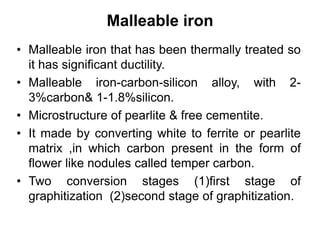
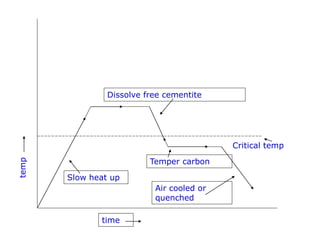
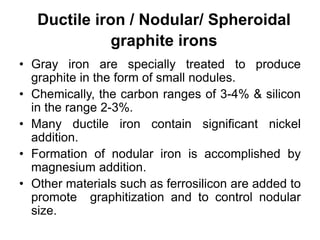
This document discusses different types of cast iron and the effects of alloying elements on steel. It describes gray cast iron, malleable cast iron, white cast iron, and ductile cast iron. For each type of cast iron, it provides details on chemical composition, microstructure, properties, and applications. It also discusses the effects that adding various elements like molybdenum, vanadium, tungsten, cobalt, silicon, copper, lead, nickel, and chromium have on the properties of steel, such as hardness, strength, corrosion resistance, and machinability.