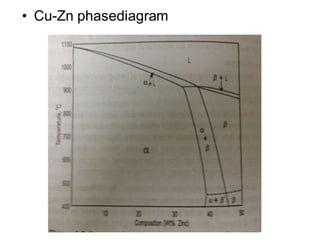
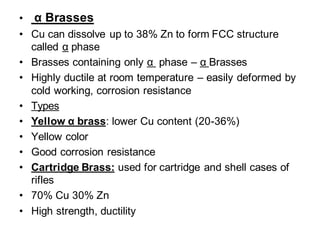
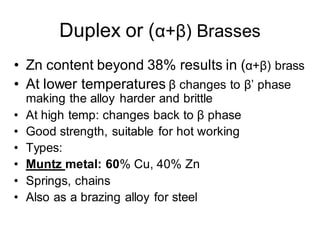
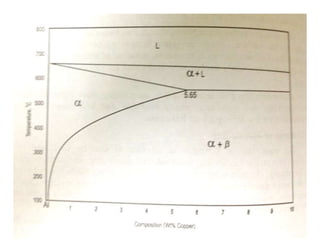
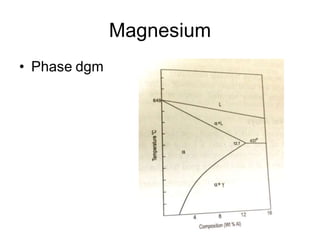
This document discusses the classification and effects of alloying elements in steels. It covers:
1. The classification of steels based on carbon content and whether they contain additional alloying elements.
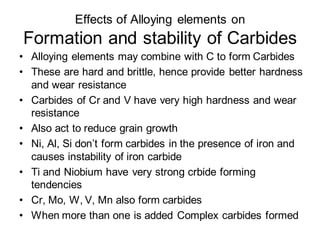
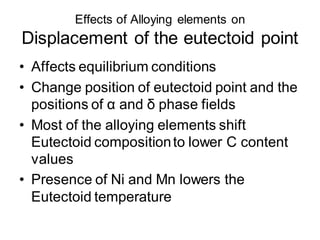
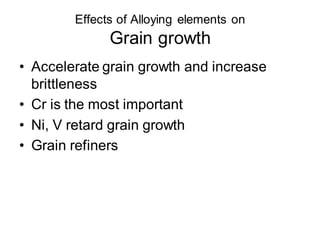
2. The effects of common alloying elements like Cr, Ni, Mo, Mn, etc. on properties like strength, hardness, corrosion resistance, and phase transformation temperatures.
3. How alloying elements are used to produce specific steel types for applications like tool steels, spring steels, rail steels, and high strength low alloy (HSLA) steels.