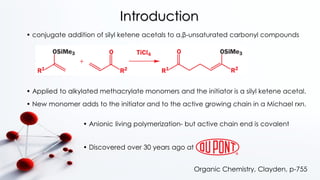
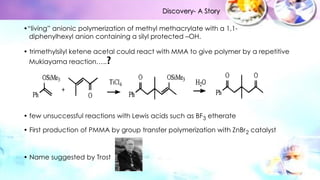
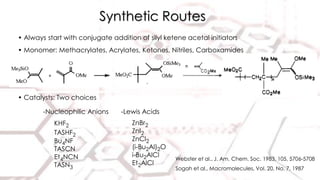
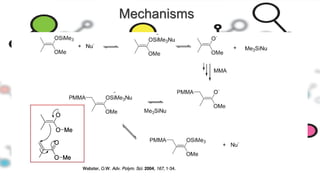
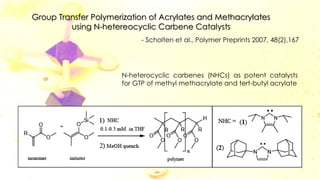
Group transfer polymerization (GTP) is a "quasi-living" polymerization technique discovered in the 1980s. It allows for the polymerization of methacrylates and acrylates at room temperature using silyl ketene acetal initiators and Lewis acid catalysts. GTP offers excellent control over polymer architecture, producing block copolymers and star polymers with low polydispersity. Recent work has applied GTP to polymerize biobased monomers like itaconic acid esters. N-heterocyclic carbene catalysts have also shown potential as alternatives to traditional bifluoride catalysts for GTP.