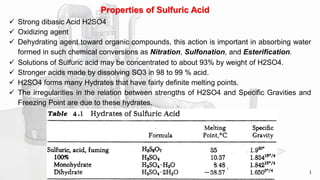
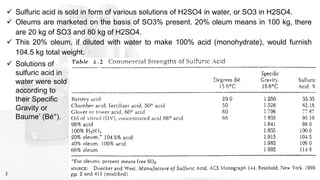
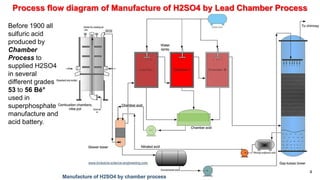
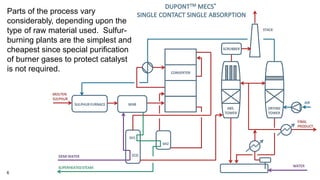
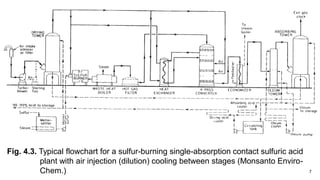
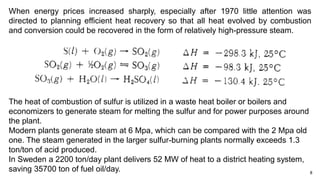
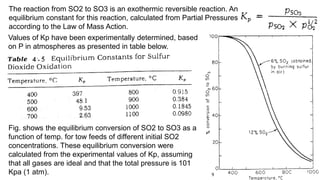
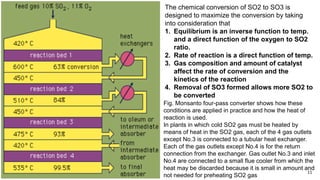
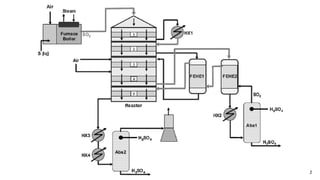
This document discusses the production of sulfuric acid through the contact process. It begins by describing the properties of sulfuric acid and how it is sold. It then discusses how earlier plants were simpler but modern plants recover energy through cogeneration. The contact process is described, including the exothermic reaction of SO2 to SO3, how temperature and equilibrium affect conversion, and the multi-pass converter design. Catalyst materials and converter design are also covered. Finally, it briefly discusses how SO3 is absorbed into sulfuric acid in the absorption tower to form oleum.