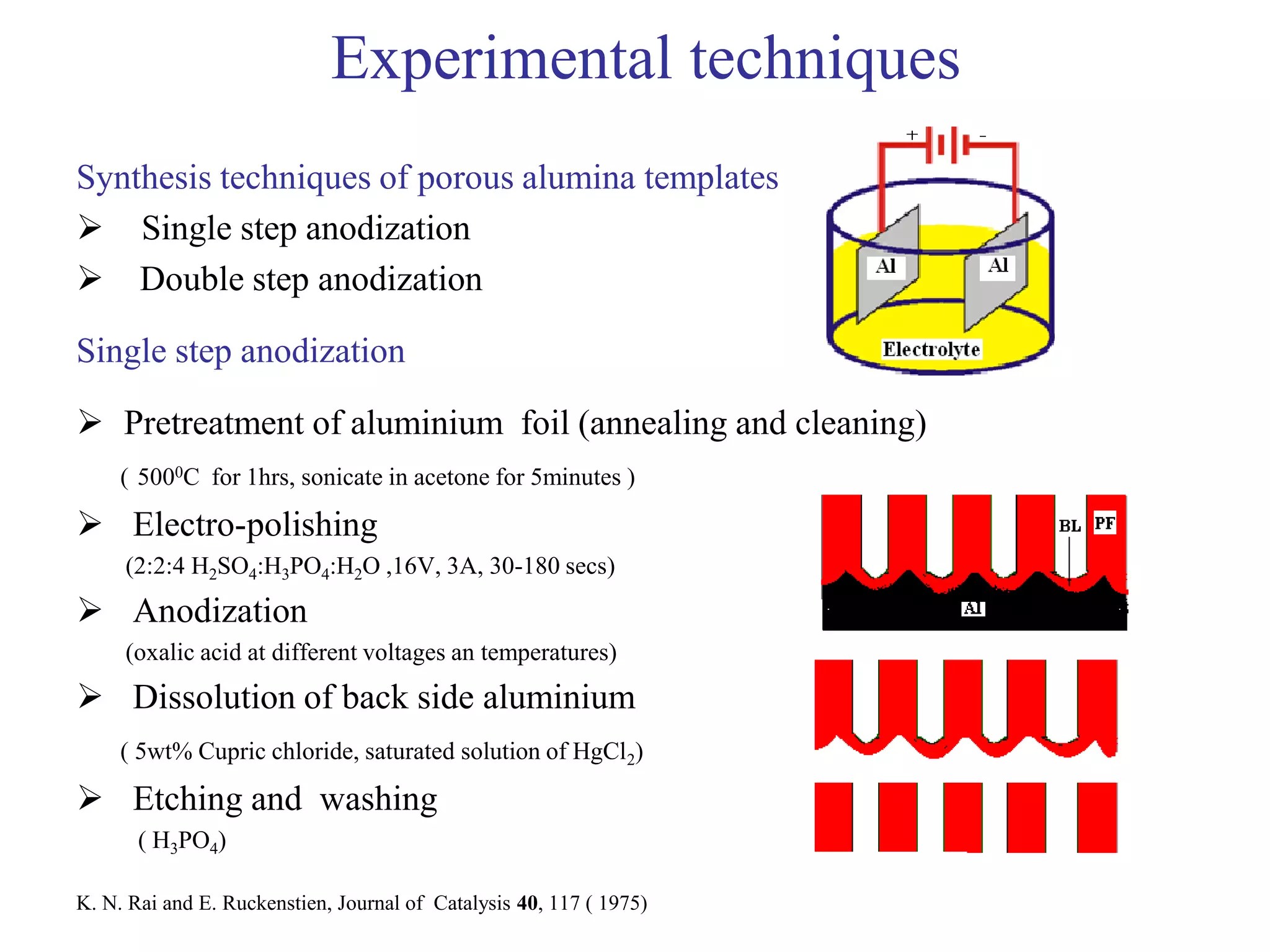
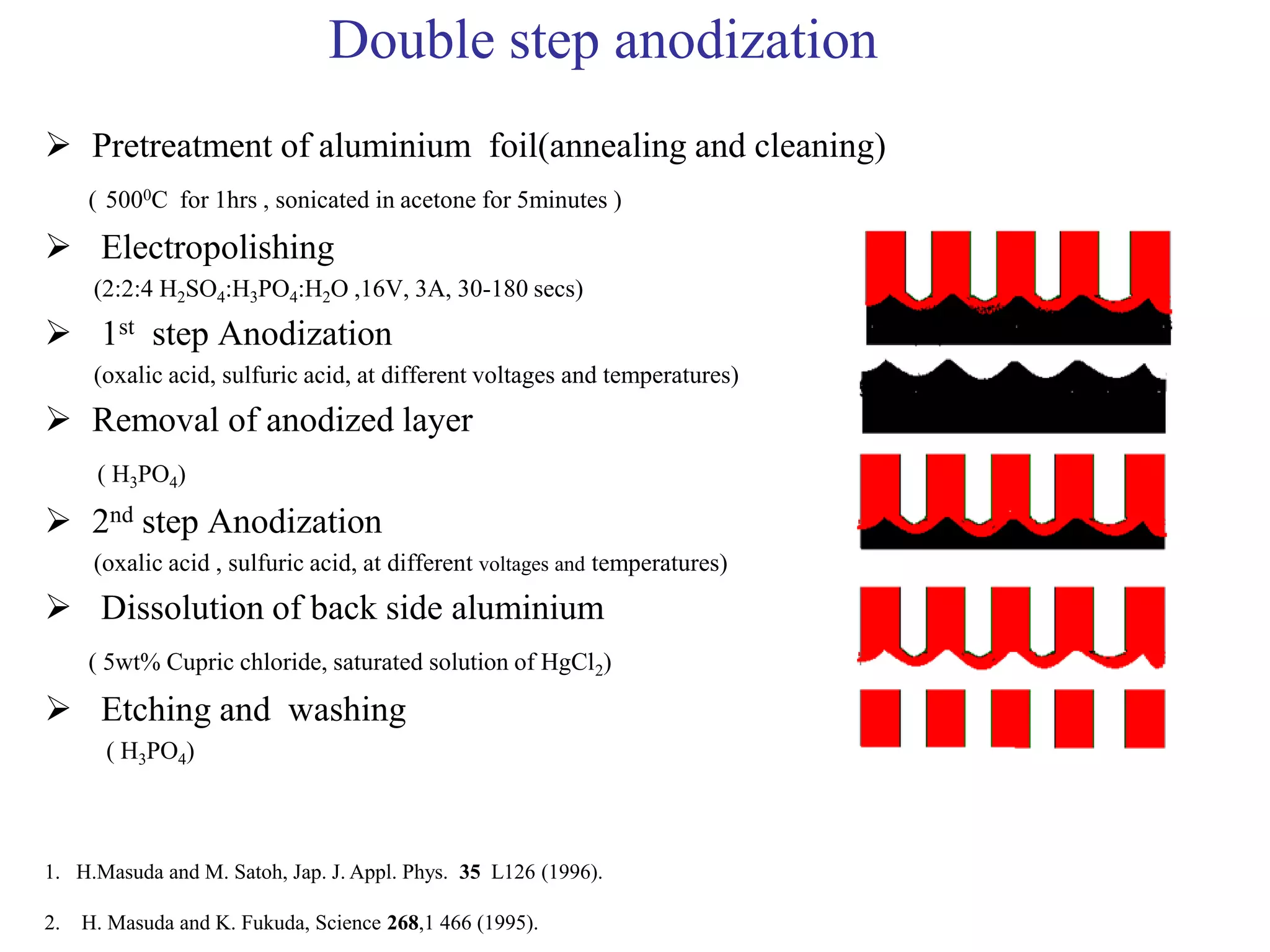
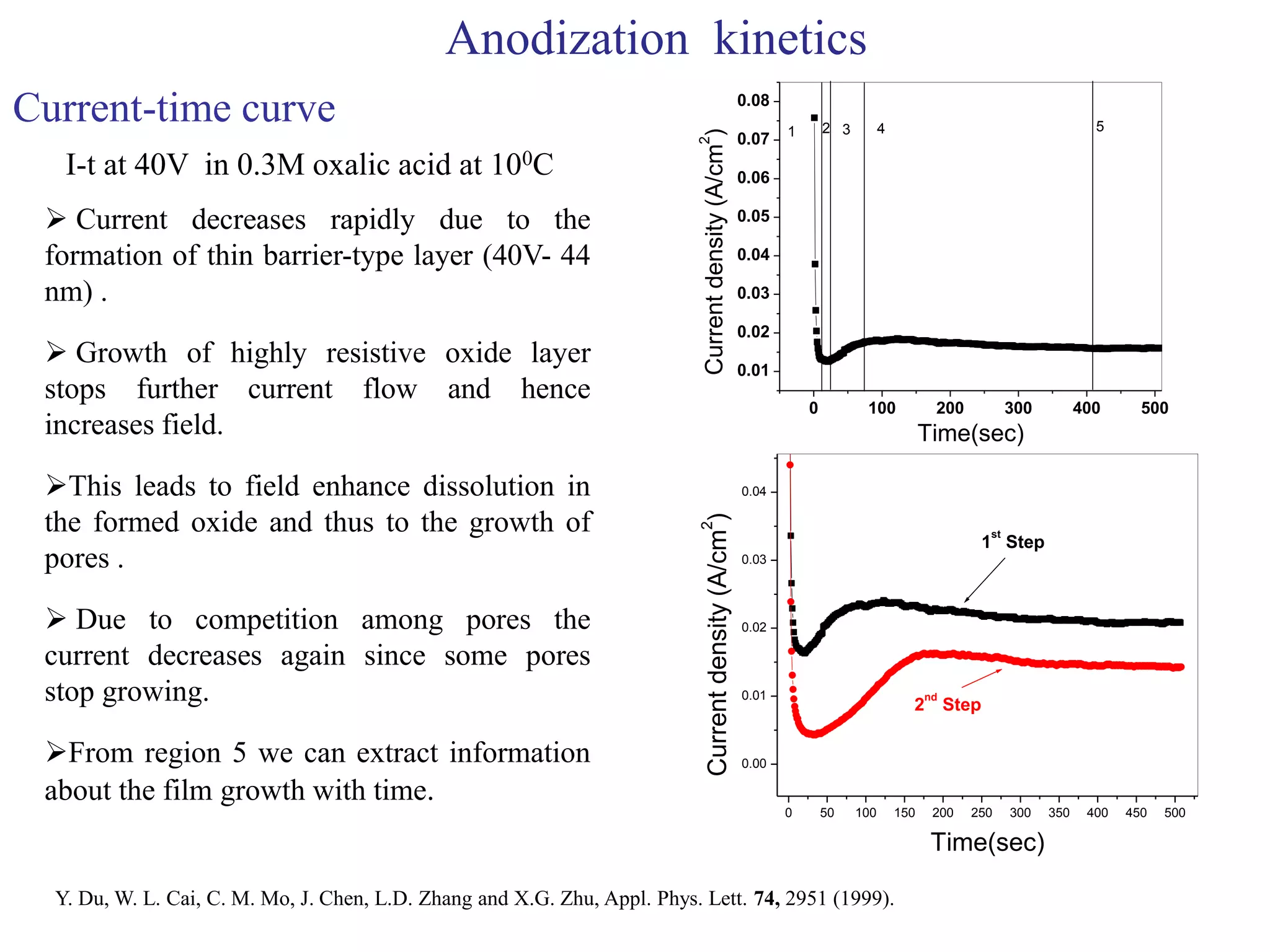
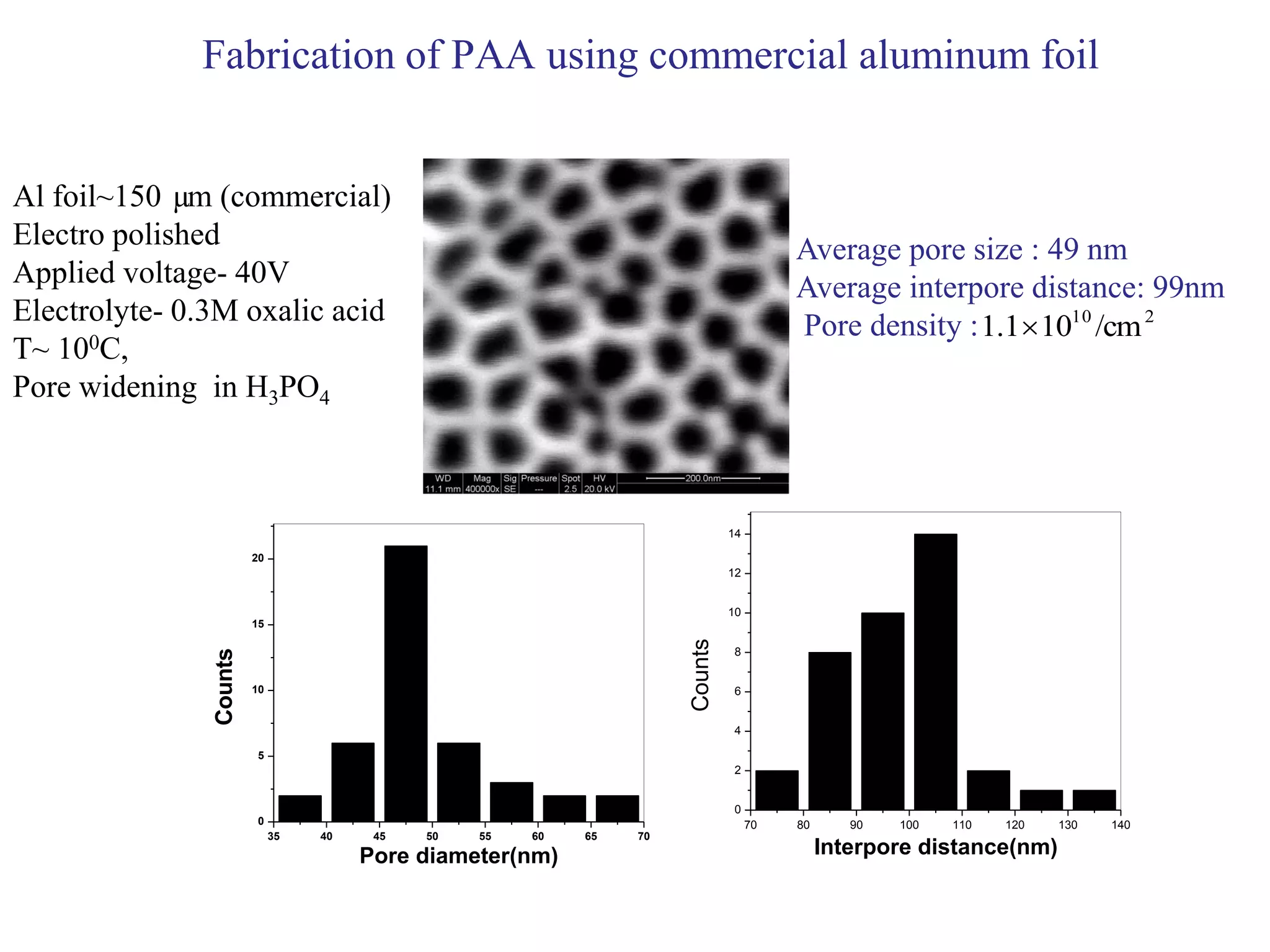
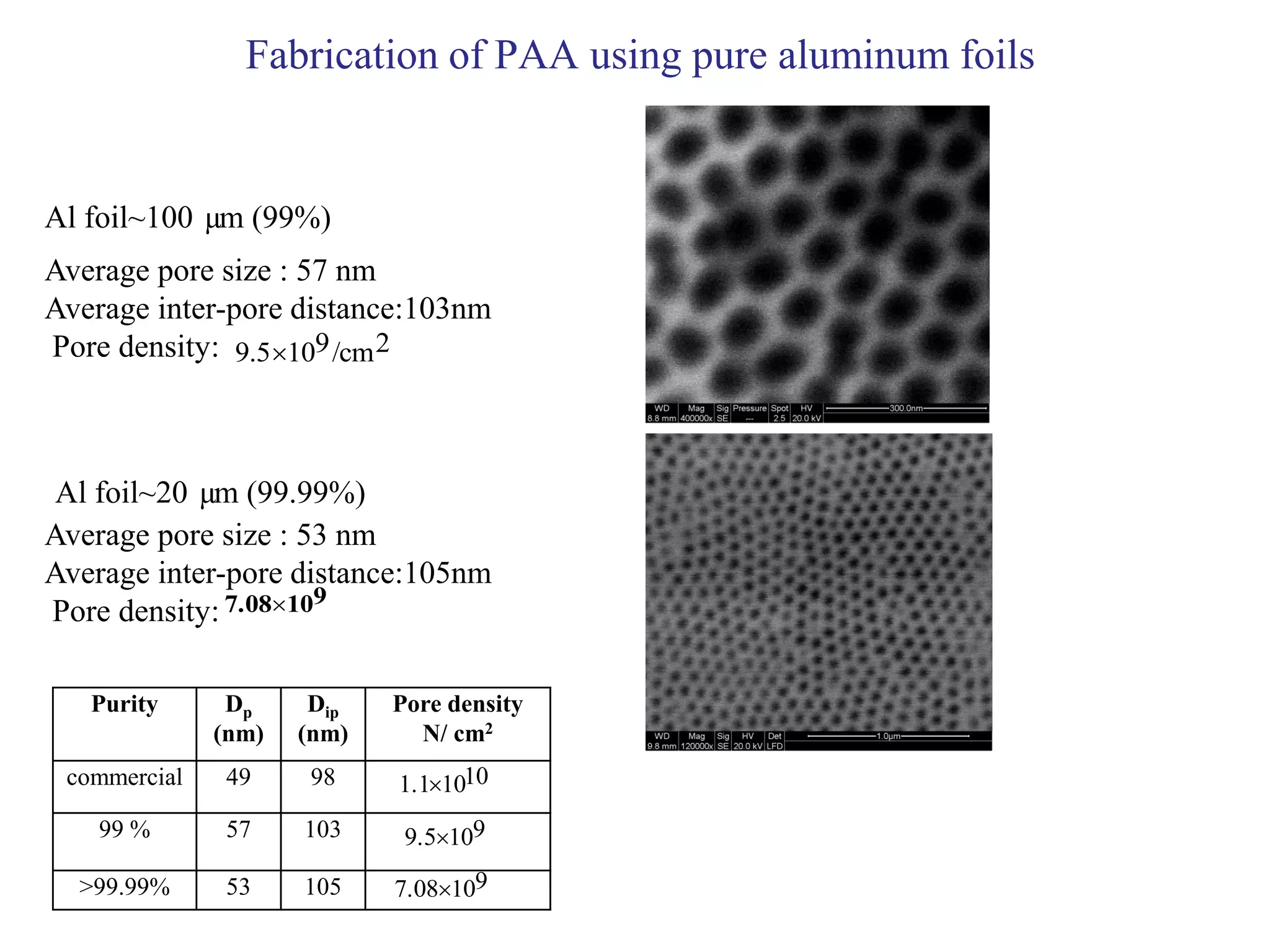
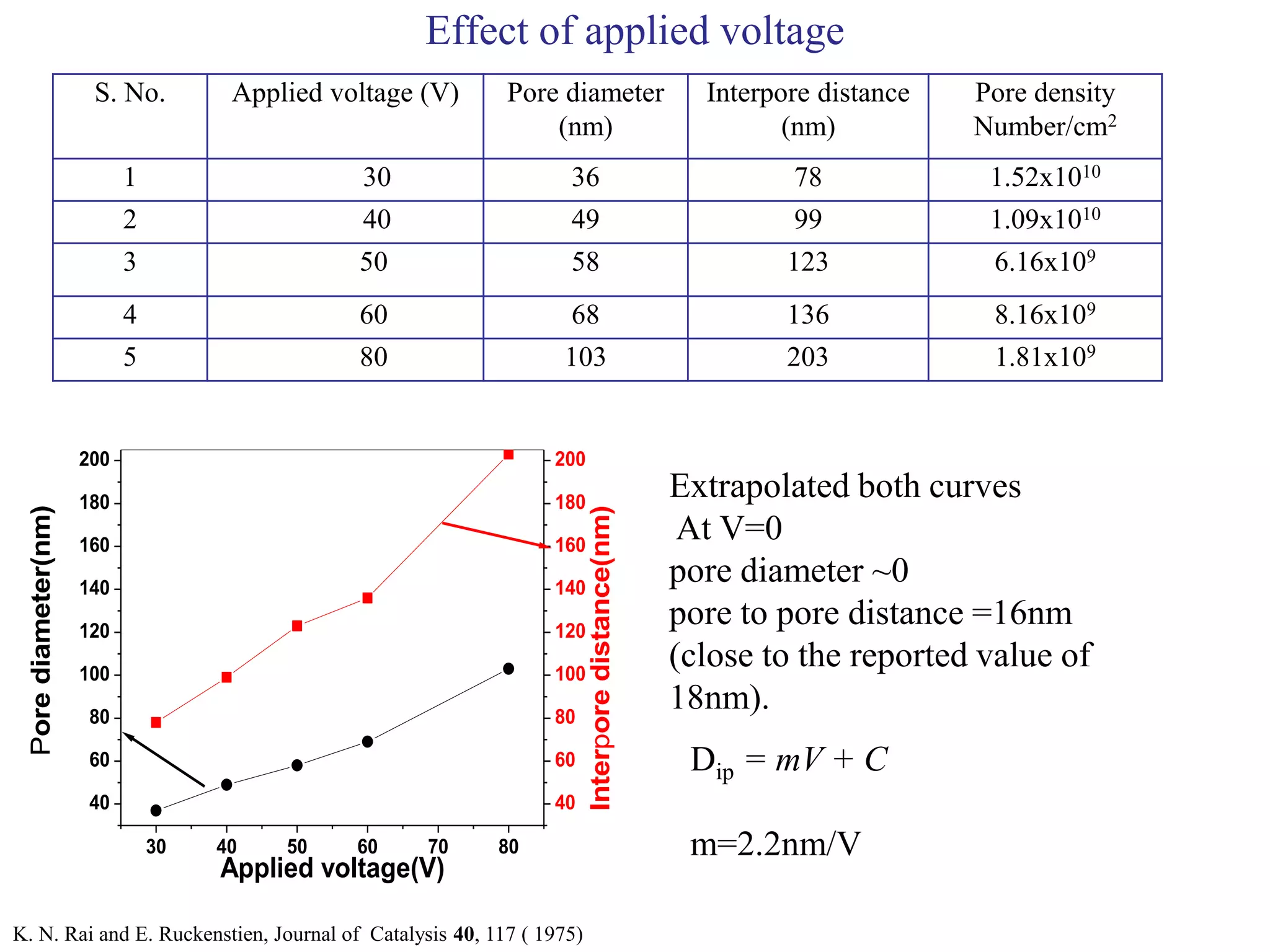
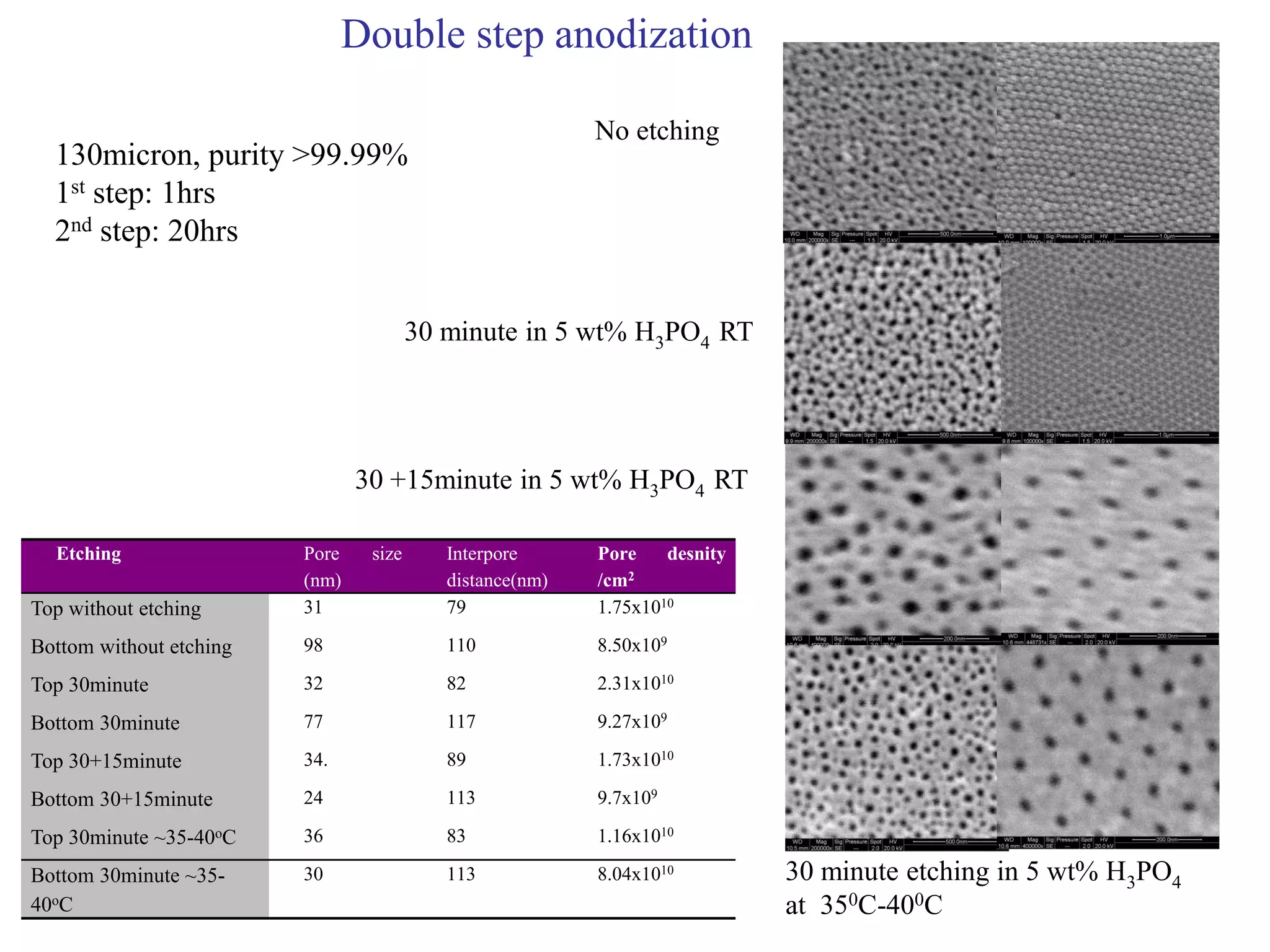
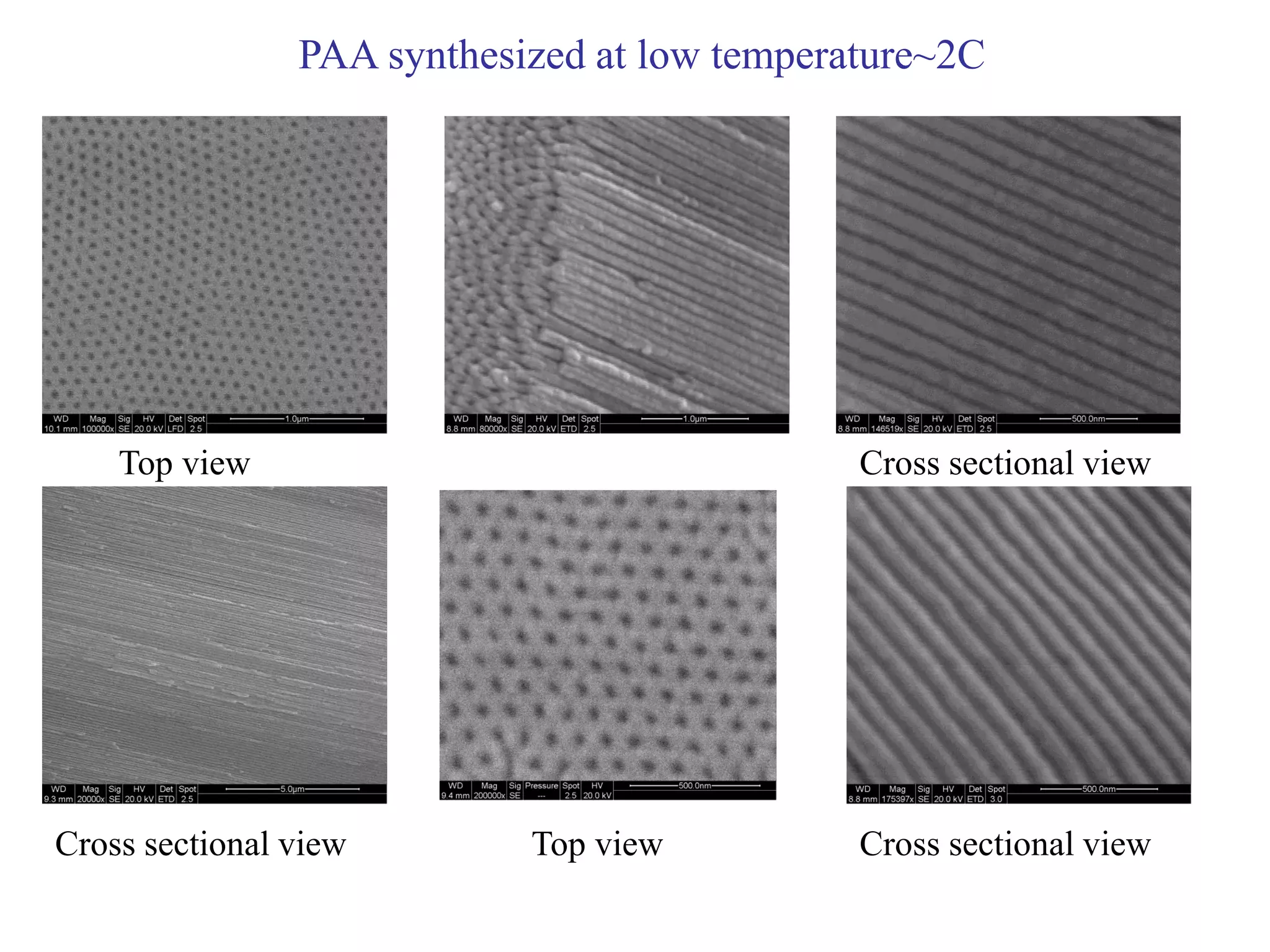
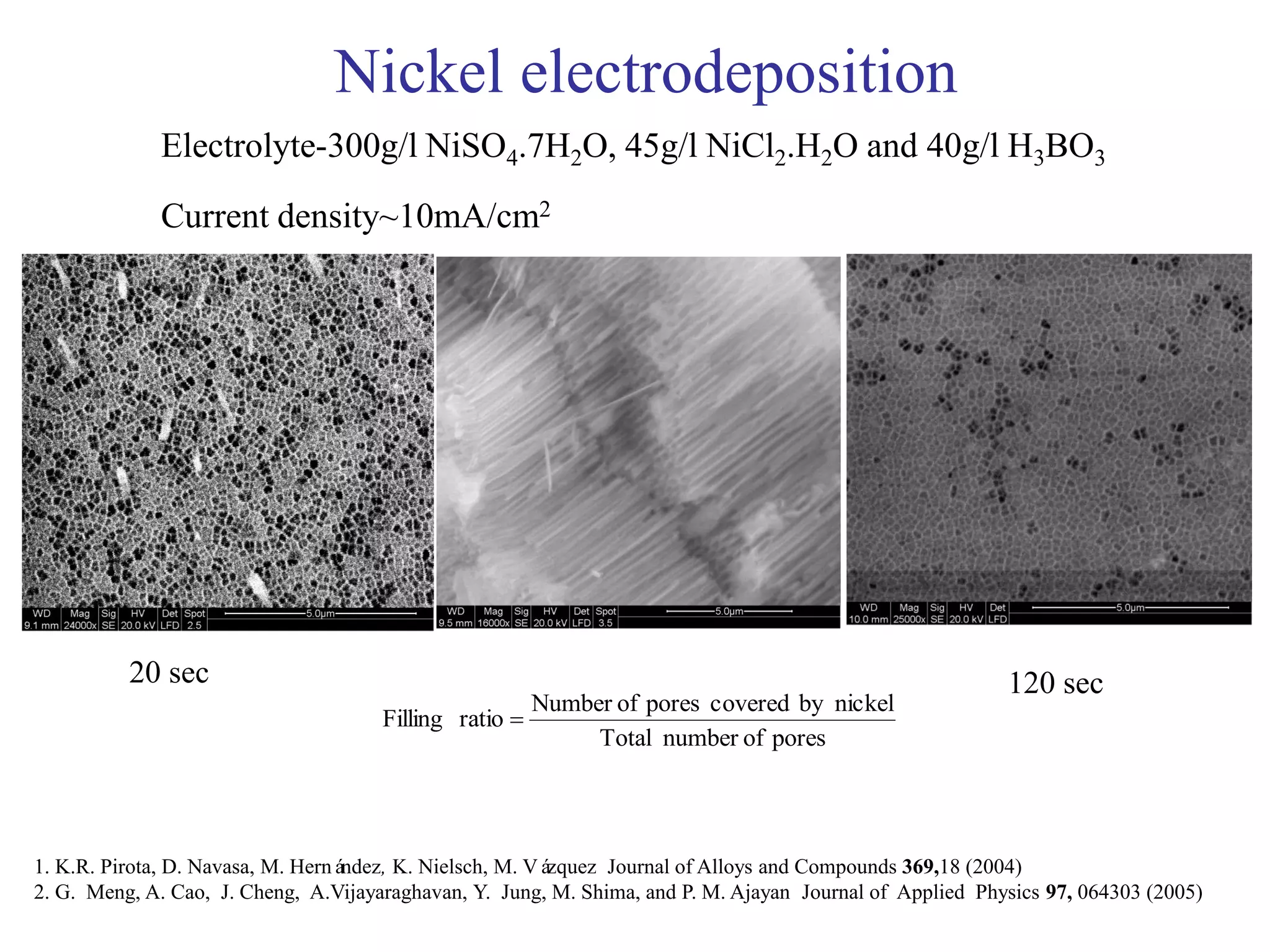
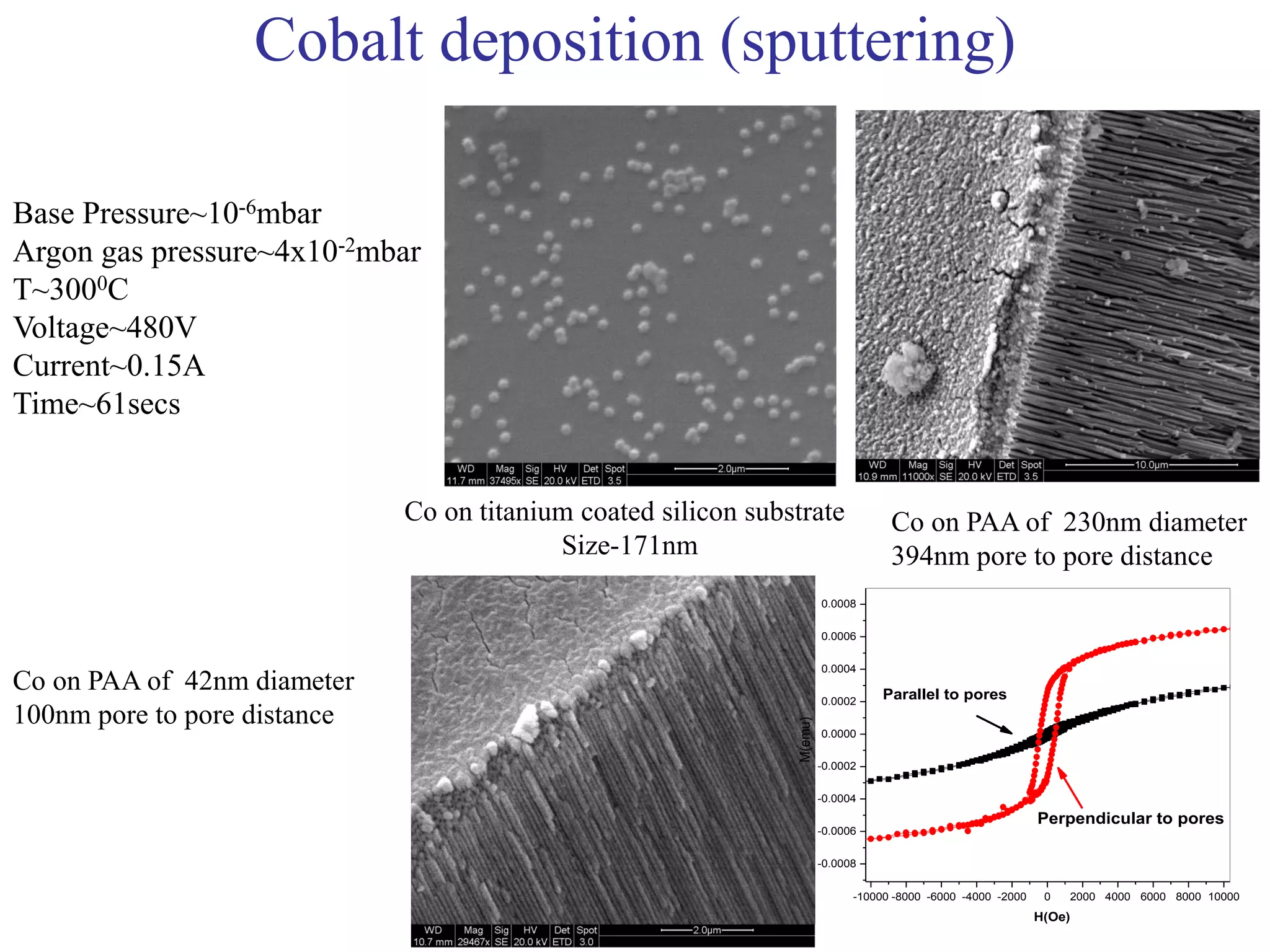
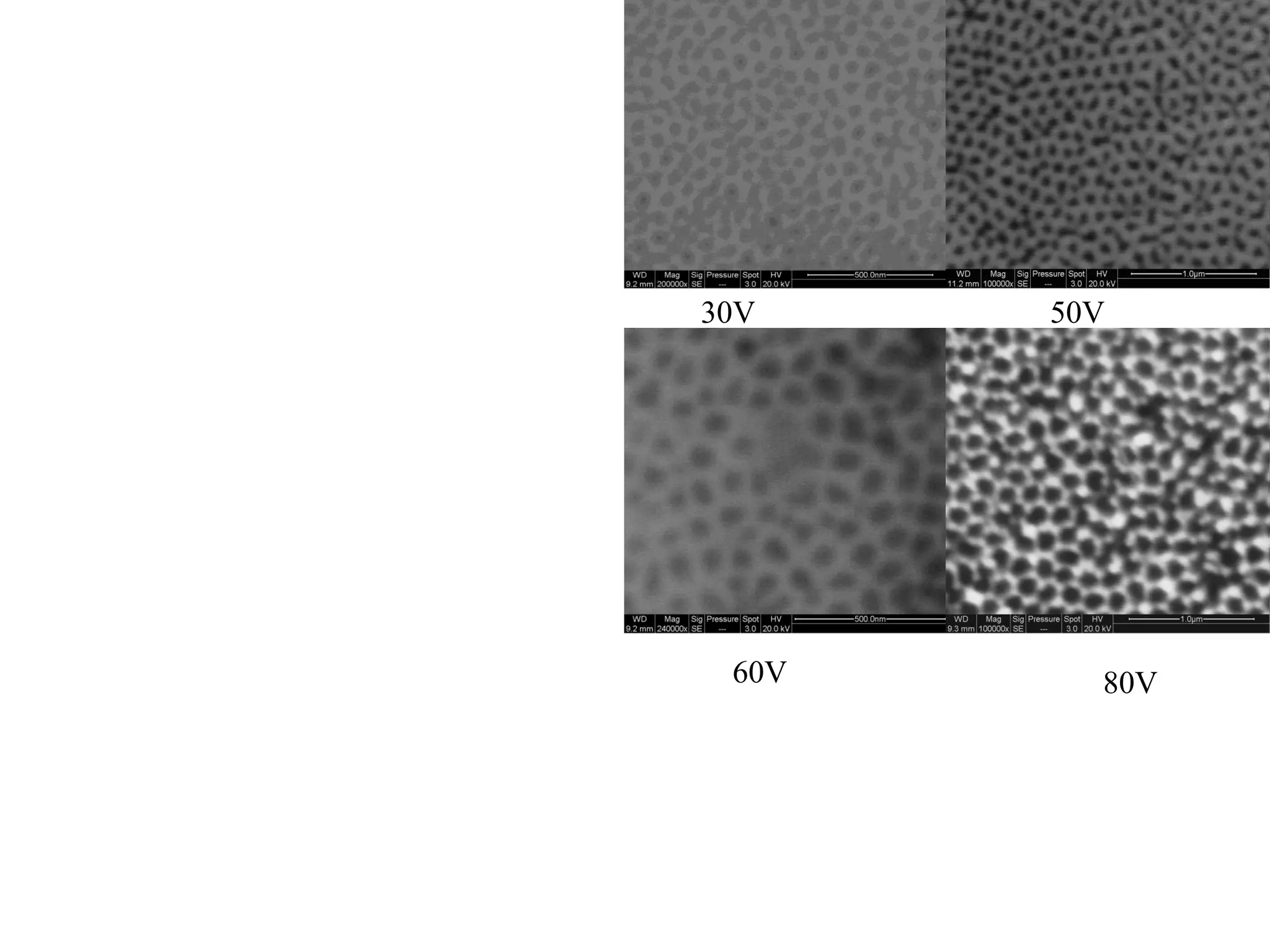
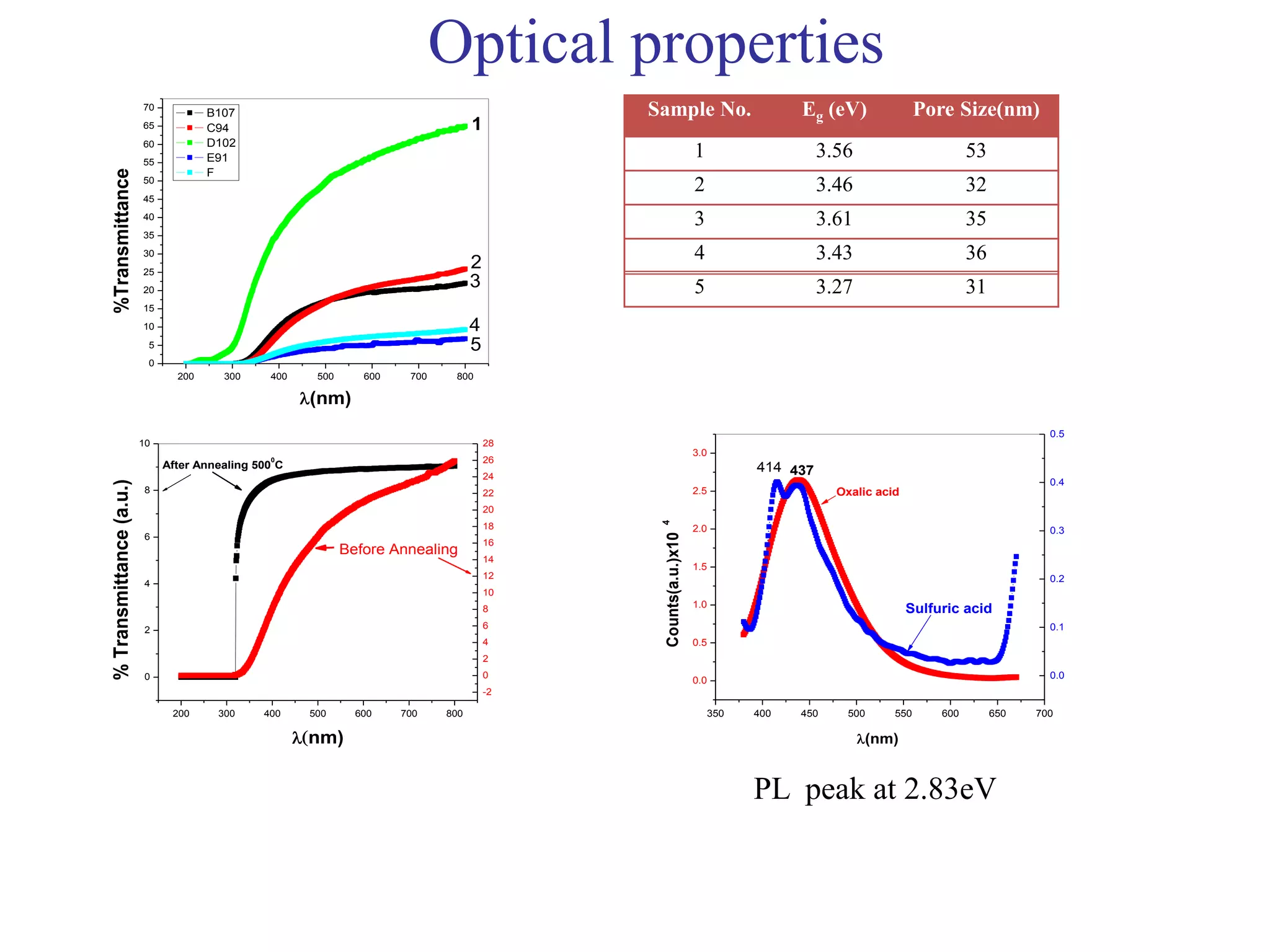
This document summarizes research on self-organized porous alumina templates and their applications in nanofabrication. It describes the experimental techniques for synthesizing porous alumina templates through anodization of aluminum foils. Factors that influence the pore size and spacing are discussed, such as applied voltage, aluminum purity, and etching time. Various deposition methods are also summarized, such as electrodeposition of nickel and sputtering of cobalt through the templates. Finally, potential applications of these templates in areas such as carbon nanotube growth and their optical properties are mentioned.