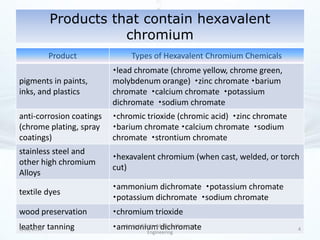
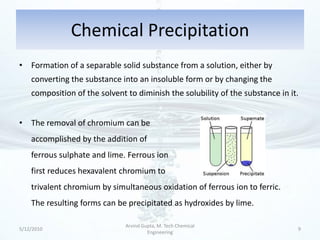
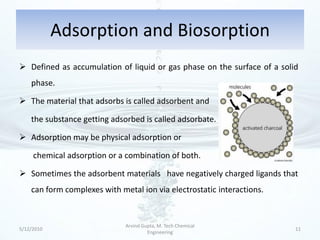
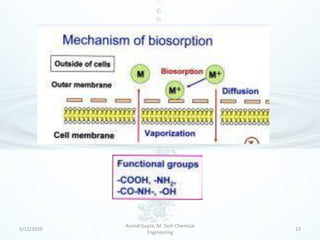
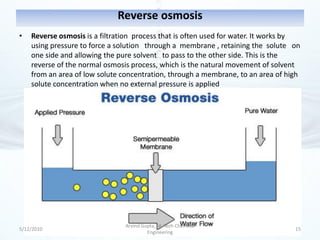
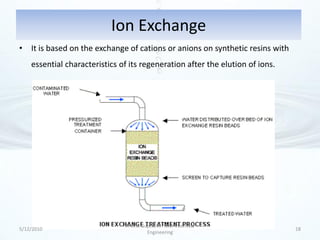
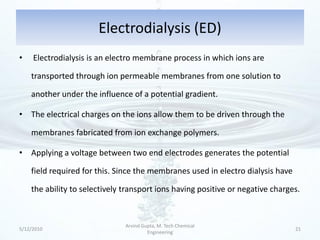
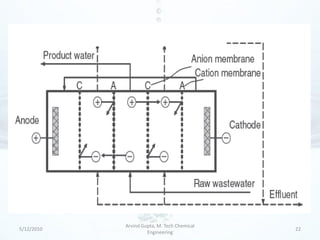
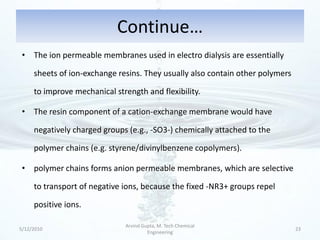
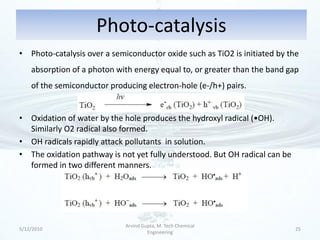
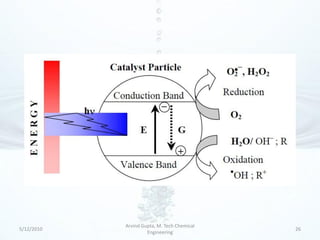
The presentation discusses methods for the removal of hexavalent chromium (Cr(VI)) from wastewater, highlighting its toxic effects and sources in various industries. Various removal techniques, including chemical precipitation, adsorption, reverse osmosis, ion exchange, electrodialysis, and photo-catalysis, are examined, each with its advantages and limitations. The conclusion emphasizes the importance of selecting a treatment method based on cost-effectiveness, technical feasibility, and the specific characteristics of the wastewater.